- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Dermatology Times
Harnessing the Potential of Placental Allografts
Placental tissues provide a promising alternative for addressing cutaneous reconstruction while maintaining the most cosmetically pleasing outcome.
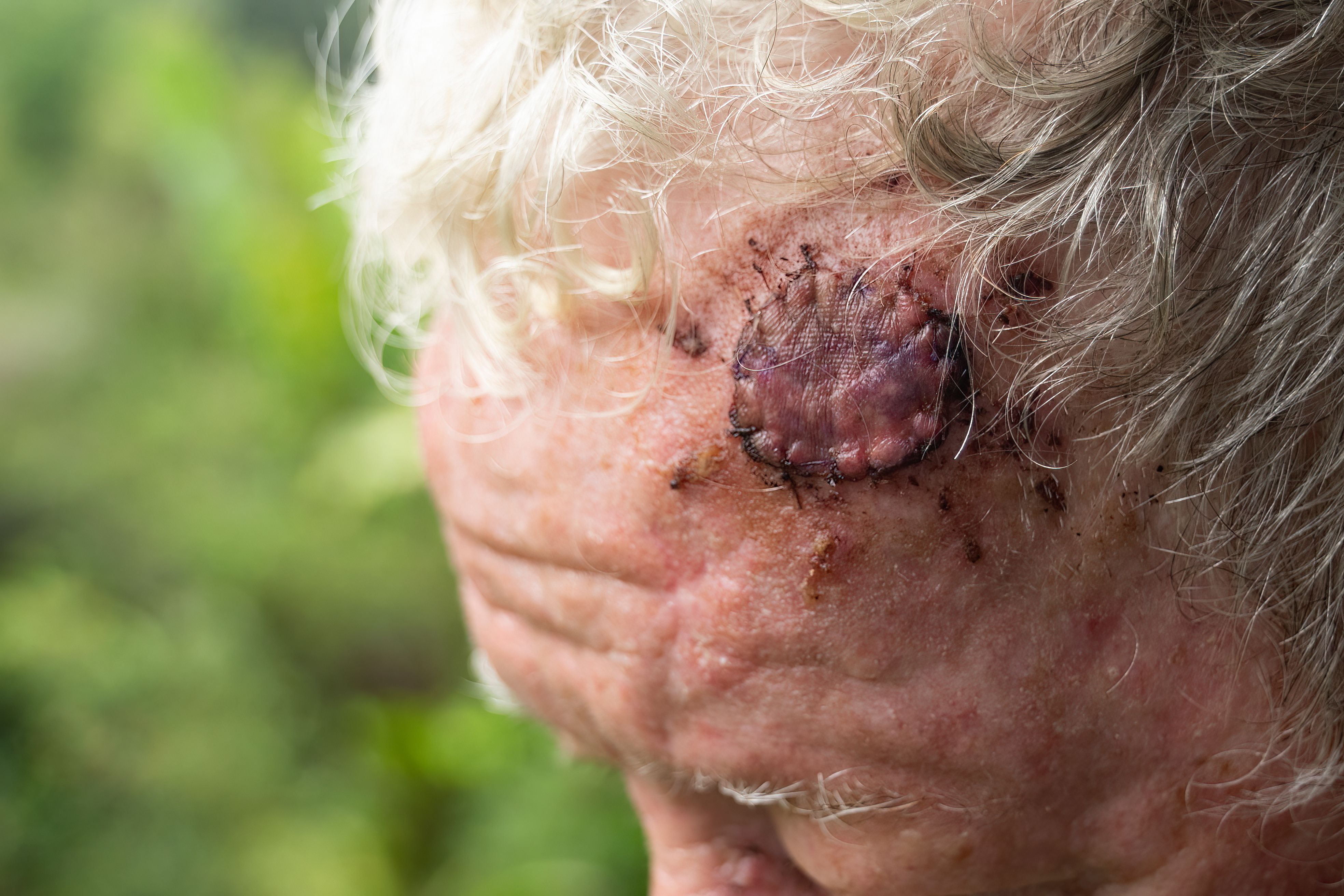
Mohs micrographic surgery (MMS) offers a precise tissue-sparing technique for nonmelanoma skin cancers with a remarkably high cure rate of up to 99%, according to the American College of Mohs Surgery.1 MMS is recommended for skin cancers located on cosmetically sensitive areas, such as the head, face, neck, hands, feet, and genitalia. It is also recommended for cases where skin cancers have recurred after prior treatment. Surgical reconstruction for defects resulting from MMS includes primary closure, flaps, grafts, and healing by second intention.1,2 Reconstruction after MMS uses autologous tissue for local flaps and full-thickness skin grafts (FTSGs).3,4 Due to the cosmetically sensitive areas targeted in MMS, the healing process is very important to maintain an aesthetic outcome. In some cases, it can take up to a year for a surgical site to fully heal.5 Potential complications from reconstruction of defects include scarring, postoperative pain, bleeding, hematoma, flap or graft necrosis, and wound infection.2 The challenges in closing postoperative defects, especially in cosmetically sensitive areas, have led to innovative approaches beyond traditional repair options, such as the recent innovation of using placental allografts for the repair of defects after MMS.
Placental Tissue in Tissue Engineering
Placental tissues provide a promising alternative for addressing cutaneous reconstruction while maintaining the most cosmetically pleasing outcome. The human placenta is composed of the outer chorion and inner amnion.6 The potential success of placental tissue grafting can be attributed to the unique properties of the amniotic membrane (AM). The AM serves as an effective scaffold for tissue engineering due to its extracellular matrix components, including collagen, fibronectin, laminin, and proteoglycans. These components facilitate cell adhesion, promoting cell proliferation and differentiation. Moreover, the AM exhibits anti-inflammatory, antifibrotic, and antiscarring properties, which contribute to a favorable environment for tissue regeneration. Its low immunogenicity reduces the risk of rejection, and the membrane’s mechanical properties provide adequate structural support. Additionally, the AM’s antimicrobial properties contribute to reduced risk of infection. Altogether, these characteristics make placental tissue grafting a promising and versatile approach for promoting tissue repair and regeneration across various medical applications.7
The Utility of Placental Allografts in Mohs
In a retrospective case-control study published in Facial Plastic Surgery & Aesthetic Medicine in 2022, Toman et al sought to assess the safety and benefits of a dehydrated human amnion/chorion membrane (dHACM) as a nonsurgical approach to cutaneous reconstruction. This study compared 286 patients who underwent MMS for defect reconstruction in visually prominent areas and who either received placental allograft or the traditional autologous tissue-based procedures: flaps and FTSGs. The investigators found that the placental allograft cases were associated with a significantly lower risk for infection (P = .004), poor scar cosmesis (P < .0001), scar revision (P < .0001), or reoperation (P = .0007). It is important to note that the placental reconstruction group did not experience a higher postoperative complication rate when compared with the autologous tissue counterpart, suggesting that dHACM has the potential to be a safe alternative to flaps and FTSGs in cosmetically sensitive repairs.4
Not all defects following MMS can be repaired immediately in the office. Some wounds may be left open to heal via secondary intention. Consequently, these cases will entail a considerably longer healing period. In a retrospective study involving 80 patients, Moradi et al compared the use of dHACM allograft to secondary intention healing in postoperative wounds following MMS. The researchers compared time to complete healing between wounds treated with dHACM allografts (n = 40) and wounds healed by secondary intention (n = 40) over several weeks. They found that the use of dHACM allograft led to significantly faster wound healing at 5.2 weeks ± 2.4 vs 6.5 weeks ± 2.7 in the control group (P = .01) for post-MMS defects in specific sensitive anatomic areas. In conclusion, they discovered that the healing time after applying dHACM allograft was 80% faster compared with healing time of wounds of a similar nature managed through secondary intention, with no noticeable difference in cosmesis. The study findings suggest dHACM as a potential alternative for postoperative wound management, highlighting the need for further studies with larger samples.8

Older individuals undergoing MMS with full-thickness defects exposing bone present specific challenges in healing, including factors such as weakened immune systems, heightened infection susceptibility, limited tolerance for bone exposure procedures, and challenges in applying dressings to difficult-to-reach wounds. A case series from Lyons et al explored the use of weekly dHACM allograft placement in 5 older patients with full-thickness MMS defects to the scalp/forehead. Weekly, the grafts were applied directly to the bone, integrating into the wound beds, followed by petroleum jelly and wound dressing. The patients would return once per week to receive placement of a new graft. This application facilitated timely wound bed granulation. The use of dHACM in wound care presented a significant advantage in streamlining post procedure care by requiring only a single office visit weekly for dressing changes and graft reapplication as opposed to the daily attention required with traditional healing by secondary intention. Lyons et al found that this innovative approach, coupled with the graft’s anti-inflammatory properties, resulted in reduced pain for patients because none of the patients required narcotics and fewer than 50% reported taking acetaminophen, which was most recommended to the author’s standard patient who underwent MMS. The decrease of pain was hypothesized to be due to both the graft’s anti-inflammatory properties as well as the physical barrier covering nerve endings. Finally, the use of dHACM eliminates the need for intrusive bone chiseling, which is traditionally used to induce pinpoint bleeding and encourage granulation. The graft’s composition includes cytokines and growth factors, aiding in a more streamlined healing process. Overall, the allografts facilitated granulation, reduced post procedure pain, and simplified wound care.9
MMS and the subsequent repair of lower eyelid surgical defects also pose distinctive challenges in reconstruction due to the inherent risk of ectropion. Addressing these challenges requires careful consideration and specialized techniques to mitigate the difficulty associated with achieving optimal outcomes. Focusing on lower eyelid defects after MMS, a case series documented by Oliver J. Wisco, DO, FAAD, presents 3 successful repairs of relatively superficial lower eyelid defects using dHACM allograft. The cases demonstrate a nonsurgical repair option with minimal pain, complications, and scarring. In conclusion, dHACM allograft is presented as an effective closure option, providing growth factors and cytokines and modulating inflammatory processes.10,11,12
Conclusion
MMS has long been a gold standard for treating cutaneous malignant tumors, offering precise excision with minimal tissue loss. However, the challenges in closing postoperative defects, especially in cosmetically sensitive areas, have led to innovative approaches beyond traditional repair options. Recent research has shown that the use of dHACM can greatly improve the outcomes of Mohs surgery. Along with successfully reconstructing cosmetically sensitive defects, dHACM allografts have been found to reduce wound healing time, improve wound care, and lower the risk of infection. Because these innovations may continue to offer additional options for healing of Mohs defects, further research and larger studies are crucial to establish their broader applicability and benefits along with consideration of the impact of the cost of these new products.
Nina Ventura is a doctor of osteopathic medicine candidate and a master of health services administration candidate at Lake Erie College of Osteopathic Medicine in Elmira, New York.
Michael Whitworth, DO, FAOCD, FAAD, is a board-certified dermatologist and Mohs micrographic surgeon at the Grekin Skin Institute and Advanced Dermatology of Michigan and is assistant program director and surgical director of the Michigan State University Beaumont Trenton Residency Program. He has no relevant disclosures.
References
- For patients. American College of Mohs Surgery. Accessed November 30, 2023. https://www.mohscollege.org/for-patients
- Bowen GM, White GL Jr, Gerwels JW. Mohs micrographic surgery. Am Fam Physician. 2005;72(5):845-848.
- Tokede O, Jadotte YT, Nkemjika S, et al. Effectiveness of Mohs micrographic surgery for nonmelanoma skin cancer: a systematic review protocol. JBI Database System Rev Implement Rep. 2017;15(3):666-675. doi:10.11124/JBISRIR-2016-002997
- Toman J, Michael GM, Wisco OJ, Adams JR, Hubbs BS. Mohs defect repair with dehydrated human amnion/chorion membrane. Facial Plast Surg Aesthet Med. 2022;24(1):48-53. doi:10.1089/fpsam.2021.0167
- Post-operative care. American College of Mohs Surgery. Accessed November 30, 2023. https://www.mohscollege.org/for-patients/about-mohs-surgery/post-operative-care
- Sridhar U, Tripathy K. Amniotic membrane graft. In: StatPearls. StatPearls Publishing; 2023. Accessed November 30, 2023. https://www.ncbi.nlm.nih.gov/books/NBK567771/
- Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99. doi:10.22203/ecm.v015a07
- Moradi S, Ormaza A, Ezra N. Dehydrated human amnion/chorion membrane allograft for postoperative wounds following Mohs micrographic surgery: a retrospective comparative evaluation. Wounds. 2023;35(9):E282-E286. doi:10.25270/wnds/23034
- Lyons AB, Chipps LK, Moy RL, Herrmann JL. Dehydrated human amnion/chorion membrane allograft as an aid for wound healing in patients with full-thickness scalp defects after Mohs micrographic surgery. JAAD Case Rep. 2018;4(7):688-691. doi:10.1016/j.jdcr.2018.03.015
- Wisco OJ. Case series: the use of a dehydrated human amnion/chorion membrane allograft to enhance healing in the repair of lower eyelid defects after Mohs micrographic surgery. JAAD Case Rep. 2016;2(4):294-297. doi:10.1016/j.jdcr.2016.06.002
- Stebbins WG, Hanke CW, Petersen J. Enhanced healing of surgical wounds of the lower leg using weekly zinc oxide compression dressings. Dermatol Surg. 2011;37(2):158-165. doi:10.1111/j.1524-4725.2010.01844.x
- Tehrani FA, Peirovi H, Niknejad H. Determination of antibacterial effect of the epithelial and mesenchymal surfaces of amniotic membrane on Escherichia coli, Staphylococcus aureus. Qom Univ Med Sci J. 2013;7(4).