- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
CME
Article
Dermatology Times
3 Things You Should Know About the Pathogenesis and Treatment of HS
Author(s):
This CME credit opportunity delves into the pathogenesis and management of hidradenitis suppurativa.
Learning Objectives
Upon successful completion of this activity, you should be better prepared to:
- Describe the pathogenic events involved in the development of hidradenitis suppurativa, including the interactive role of inflammation and the cutaneous microbiome
- Interpret clinical findings in support of a diagnosis of hidradenitis suppurativa
- Evaluate the potential of emerging therapies to address unmet needs in patients with hidradenitis suppurativa based on knowledge of pathophysiological disease mechanisms and therapeutic drug targets
This activity was written by PER® editorial staff based on an online activity developed with Maloney, Block, and Chao.

Acknowledgment of Educational Grant Support
This activity is supported by an educational grant from Novartis Pharmaceuticals.
ACTIVITY
Hidradenitis suppurativa (HS) is a chronic condition that presents as inflammatory nodules or abscesses in regions of the skin bearing apocrine glands. HS progression leads to the formation of deep abscesses, sinus tracts, fistulae, and scarring, causing significant pain and limiting mobility.1 No uniform treatment strategy for HS exists due to its unpredictable disease course. Various topical and systemic pharmacologic therapies, as well as surgical interventions, are used depending on disease presentation and severity.2 The recent FDA approval of secukinumab, an interleukin (IL)-17A inhibitor, offers patients and providers a new management option for this challenging condition.3
Here are 3 things you should know about the pathogenesis and management of HS.
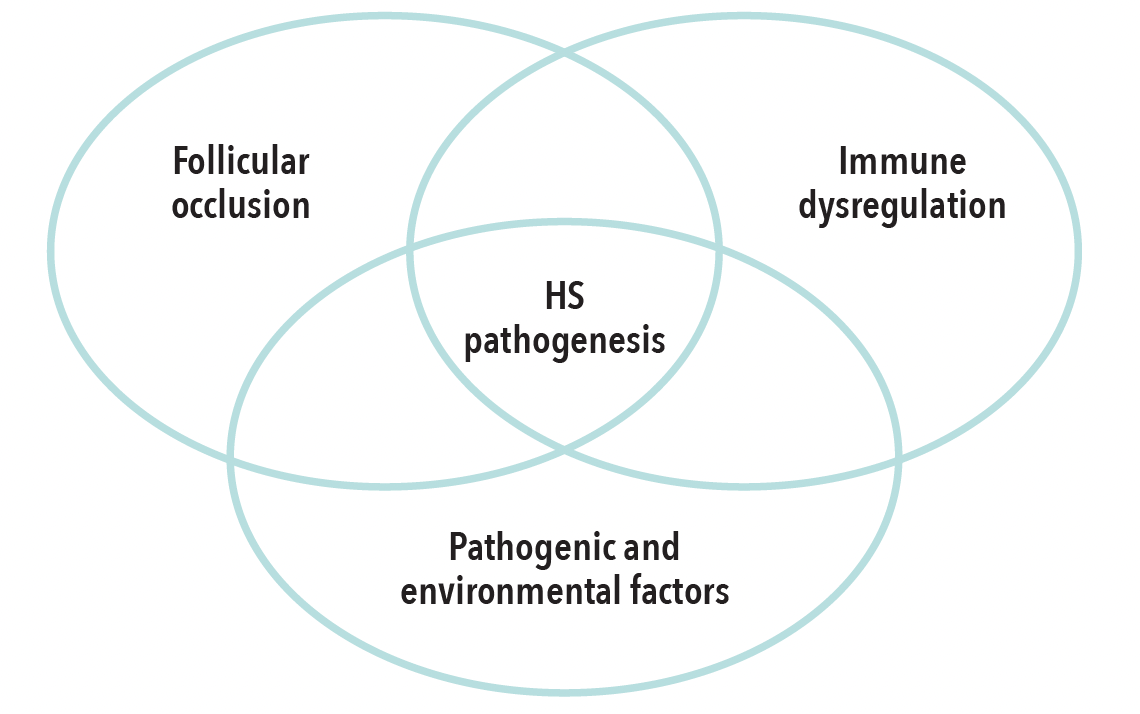
1. HS pathogenesis involves hair follicle occlusion and immune dysregulation.
The HS disease process is driven by occlusion and inflammation of the hair follicle, in combination with dysregulation of the innate and adaptive immune system.4 Secondary pathogenic and environmental factors also contribute to lesion formation and progression.4,5
Although the primary cause of follicular keratin occlusion is unclear, epidermal hyperplasia caused by innate immune dysregulation leads to the formation of cysts.6-9 Cystic rupture frees the keratin fibers and releases commensal bacteria into the dermis, amplifying local inflammation.10,11 Keratin fibers and other tissue destruction byproducts are recognized by macrophages and dendritic cells.6 These interactions lead to increased production of proinflammatory cytokines like tumor necrosis factor alpha (TNF-α). TNF-α-mediated activation of T-cells results in the release of proinflammatory molecules, including interferon gamma and IL-17. Elevated levels of IL-17 in lesional, perilesional, and unaffected skin also suggest a role of subclinical inflammation prior to lesion formation. High levels of IL-23, which drives IL-17 production, in HS lesions also implicate the IL-23/IL-17 axis in HS pathogenesis.12
The HS pathogenesis is complex and multifaceted.
2. HS diagnosis and staging considers the pattern and chronicity of lesions.
The diagnostic criteria for HS include characterization and distribution of lesions, as well as chronicity and recurrence of symptoms.13 HS lesion morphology includes papules, nodules, abscesses, sinus tracts, fistulas and scarring. Lesions persist for at least 3 months and are distributed in axillary, inframammary, groin, perineal and gluteal regions. Additionally, more than 2 lesions occur within a 6-month period.13
Accurate disease staging is needed for selection of an appropriate management plan. Although other scoring systems are also used in clinical practice, the Hurley staging system is most commonly used to determine HS severity. Hurley stage I indicates mild disease, with 1 or more nodules and/or abscesses. Hurley stage II, or moderate HS, involves recurring nodules and/or abscesses together with scarring and sinus tracts separated by normal skin. With Hurley stage III, sinus tracts and abscesses interconnect, and scarring affects an entire area.1 Hidradenitis Suppurativa Clinical Response (HiSCR) is a clinical treatment end point defined as lower count of abscesses and inflammatory nodules and no increase in abscesses or draining fistulae versus baseline.14
Many HS staging systems do not account for pain or patient burden when assessing disease severity. The visual analog scale for pain and the Dermatology Life Quality Index can help to gauge the impact of disease on daily life.15 Ultrasonography has also emerged as a diagnostic technique that can be more effective in identifying fistulous tracts and signs of active inflammation.16

3. New and emerging biologic immunomodulators provide more options for targeted therapy.
Appropriate medical management of HS depends on disease severity. Topical therapies may be sufficient for mild cases, but systemic therapy or surgical treatment may be needed for more severe disease.13 For moderate-to-severe HS, targeted immunomodulatory therapies are becoming more available.
Secukinumab is a human monoclonal antibody that targets IL-17A administered by subcutaneous injection. Two pivotal phase 3 trials, SUNSHINE (NCT03713619) and SUNRISE (NCT03713632), investigated this therapy for adult patients with moderate-to-severe disease (N = 545).17 Patients were randomized to receive 300 mgsecukinumab every 2 or 4 weeks, or placebo, after weekly loading doses. The primary end point, HiSCR50, was defined as a 50% decrease in abscess and inflammatory nodule count from baseline with no increase in the number of abscesses or draining tunnels.17 At week 16, a significant proportion of patients on the study drug achieved HiSCR50 versus placebo for those on a 2-week dosing interval (SUNSHINE: 44.5% vs 29.4% [P < .05]; SUNRISE: 38.3% vs 26.1% [P < .05]). A greater proportion of those on the 4-week dosing interval (SUNSHINE: 41.3% vs 29.4% [P > .05]; SUNRISE: 42.5% vs 26.1% [P < .05]) reached HiSCR50 at week 16.18 At week 52, the proportion of patients achieving HiSCR50 improved for the 2-week regimen (SUNSHINE: 56.4%; SUNRISE: 65.0%) and the 4-week regimen (SUNSHINE: 56.3%; SUNRISE: 62.2%).19 Safety of secukinumab was consistent with previous reports, with no new safety signals identified.17
Secukinumab was approved by the FDA in October 2023 as first-in-class option to treat moderate-to-severe HS in adults.18
Bimekizumab is an anti-IL-17A and anti-IL-17F monoclonal antibodydelivered by subcutaneous injection.20 Two double-blind, placebo-controlled phase 3 trials, BE HEARD I (NCT04242446; N=505) and BE HEARD II (NCT04242498; N=509) investigated bimekizumab in patients with moderate-to-severe HS. The trials consisted of initial (weeks 0 to 16) and maintenance (weeks 16 to 48) phases. Patients were divided into 4 treatment arms: A) bimekizumab every 2 weeks, B) bimekizumab every 2 weeks, then every 4 weeks after week 16; C) bimekizumab every 4 weeks, and D) placebo every 2 weeks, then bimekizumab every 2 weeks after week 16. At week 16, pooled data indicated that more patients achieved HiSCR50 at week 16 vs placebo (A: 58%; B: 55.9%; C: 56.1%; D: 33.4%). At week 48, 70.5% of patients who switched from placebo to the study drug achieved HiSCR50. Safety was consistent with previous studies, with no new safety signals occurring.21-23
New and emerging HS therapies target IL-17.

Key References
12. Del Duca E, Morelli P, Bennardo L, Di Raimondo C, Nistico SP. Cytokine pathways and investigational target therapies in hidradenitis suppurativa. Int J Mol Sci. 2020;21(22):8436. doi:10.3390/ijms21228436
17. Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet. 2023;401(10378):747-761. doi:10.1016/S0140-6736(23)00022-3
20. Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
For a full list of references, visit: https://gotoper.com/sdpa23hs-postref
Claim Your CME Credit at: https://gotoper.com/sdpa23hs-postref
Faculty, Staff, and Planners’ Disclosures
In accordance with ACCME Guidelines, PER® has identified and resolved all conflict of interest for faculty, staff, and planners prior to the start of this activity by using a multistep process.
Disclosures (Maloney): Consultant: Advisory boards with AbbVie;Arcutis Biotherapeutics; Bristol Myers Squibb; Janssen; Leo Pharma; Novartis.Other: DPAF trustee at-large; HS in-depth speaker through HS Foundation; SDPA membership chair. (Block):Consultant:Advisory boards with Almirall, Bristol Myers Squibb, Castle Biosciences, Dermavant Sciences,Encore, EPI, Galderma, Janssen Pharmaceuticals, LEO Pharma, Novartis Pharmaceuticals, Promius. Speakers’ Bureau: Bayer, Bristol Myers Squibb, LEO Pharma, Mayne Pharma,Pharmaderm, Sun PharmaceuticalIndustries, OrthoDerm. (Chao):Consultant:Advisory boards with AbbVie, Amgen, Arcutis Biotherapeutics, Dermavant Sciences, Janssen Pharmaceuticals, UCB. Speakers’ Bureau: AbbVie, Amgen, Arcutis Biotherapeutics, Dermavant Sciences, Incyte, Janssen Pharmaceuticals, UCB.





