- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
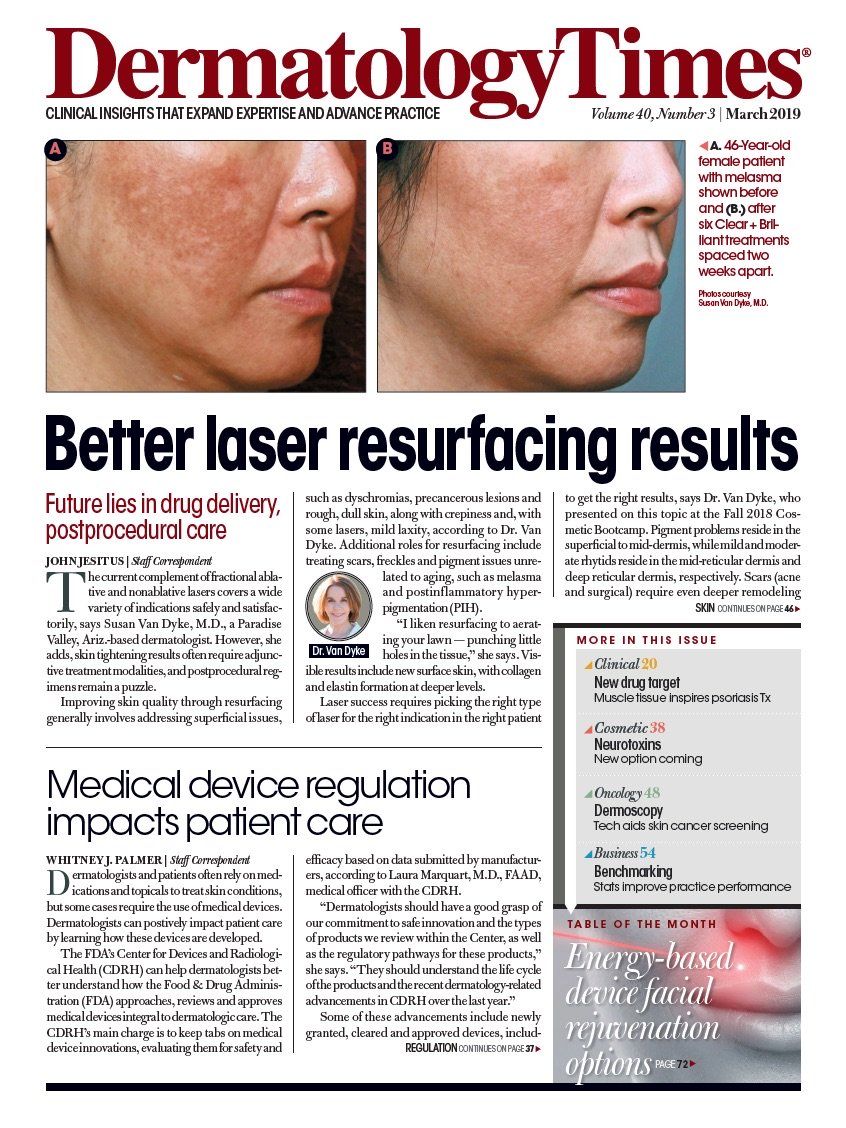
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Possible tool to quickly assess BRAF, NRAS status
Author(s):
Cell-free circulating DNA (cfDNA) can provide a useful snapshot of the BRAF and NRAS genotype of melanoma patients’ tumor tissue and act as a useful surrogate to assessing tissue biopsies for the markers, a recent study suggests.
Cell-free circulating DNA (cfDNA) can provide a useful snapshot of the BRAF and NRAS genotype of melanoma patients’ tumor tissue and act as a useful surrogate to assessing tissue biopsies for the markers, a recent study suggests. (JimVallee - stock.adobe.com).
Cell-free circulating DNA (cfDNA) can provide a useful snapshot of the BRAF and NRAS genotype of melanoma patients’ tumour tissue and act as a useful surrogate to assessing tissue biopsies for the markers, a study published in Oncotarget suggests.i
Detection of BRAF mutations is a pre-requisite for treatment with BRAF inhibitors, and detection of a mutated NRAS oncogene is a biomarker of poor outcome and resistance to treatment with BRAF inhibitors.
Several methods to detect BRAF and NRAS mutations in formalin-fixed paraffin embedded (FFPE) samples are currently available in molecular pathology laboratories worldwide, but polymerase chain reaction (PCR) based techniques require a dedicated infrastructure, which is not always present in pathology laboratories.
Liquid biopsy in metastatic melanoma has emerged as an alternative tool that is complementary to tumor biopsies for detection of 'druggable” molecular alterations, with several studies demonstrating that circulating cell-free DNA (cfDNA) represents genetic information from the whole tumor genome and can provide evidence of the clonal evolution and tumor heterogeneity in several types of cancer, including melanoma.
Researchers at the Université Côte d’Azur in France assessed the sensitivity and specificity of the fully automated ready-to-use Idylla™ PCR-based system in identifying BRAF V600 and NRAS mutations in plasma samples from 19 patients with stage IV metastatic melanoma at baseline and during the course of treatment.
The cfDNA genotype obtained with Idylla was compared to the results obtained with matched-tumor tissue obtained via FFPE and to clinical outcome.
At baseline, nine (47%) of the 19 patients harbored a BRAFV600 mutation in their cfDNA and a NRAS mutation was detectable with plasma in 15% of patients before treatment. Two months after targeted treatment with a BRAF inhibitor the BRAFV600 mutant cfDNA was undetectable in all patients and three were disease-free. Sensitivity and specificity were 80% and 89% for BRAF status, and 79% and 100% for NRAS status in pretreatment cfDNA assessed using the Idylla™ PCR-based system compared to results obtained with a tissue test.
Marius Ilie, from the Laboratory of Clinical and Experimental Pathology, Pasteur Hospital, in Nice, says: “As only patients whose tumors harbor the ‘druggable’ mutation will benefit from a targeted treatment, there is strong need for reliable, fast, and easy-to-use detection of mutations. Furthermore, a personalized treatment scheme requires monitoring of the tumor’s genomic status.”
He adds: “We demonstrated that the detection of the BRAF and NRAS cfDNA status with the Idylla™ assay is feasible in a routine manner in a pathology laboratory, before and after systemic treatment of patients with metastatic melanoma. The assay reached acceptable concordance when compared with standard molecular analyses with matched tumor tissue.”
The Idylla™ system offers a fast and easy-to-handle integrated “sample-to-result” approach with results available in less than 2 hours after blood taking, including plasma, preparation, whereas other sequencing approaches requiring samples to be manually handled can have turnaround times of several days, he explains
The Idylla™ system could also be implemented in a standard pathology laboratory, Ilie says, and the approach “could offer a good alternative to a surgical biopsy in fragile patients or patients with inaccessible metastatic sites to assess ‘druggable’ molecular alterations,” and “would be of particular interest for patients with a new metastatic melanoma, which often evolve rapidly and require urgent identification of the mutation status.”
“Large prospective clinical studies are needed to evaluate the medical impact of cfDNA-guided decisions,” he says.
Reference:i Long-Mira E, Ilie M, Chamorey E, Leduff-Blanc F, Montaudié H, Tanga V, et al. Monitoring BRAF and NRAS mutations with cell-free circulating tumor DNA from metastatic melanoma patients. Oncotarget. 2018 Nov 16;9(90):36238-36249. doi: 10.18632/oncotarget.26343. eCollection 2018 Nov 16.