- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
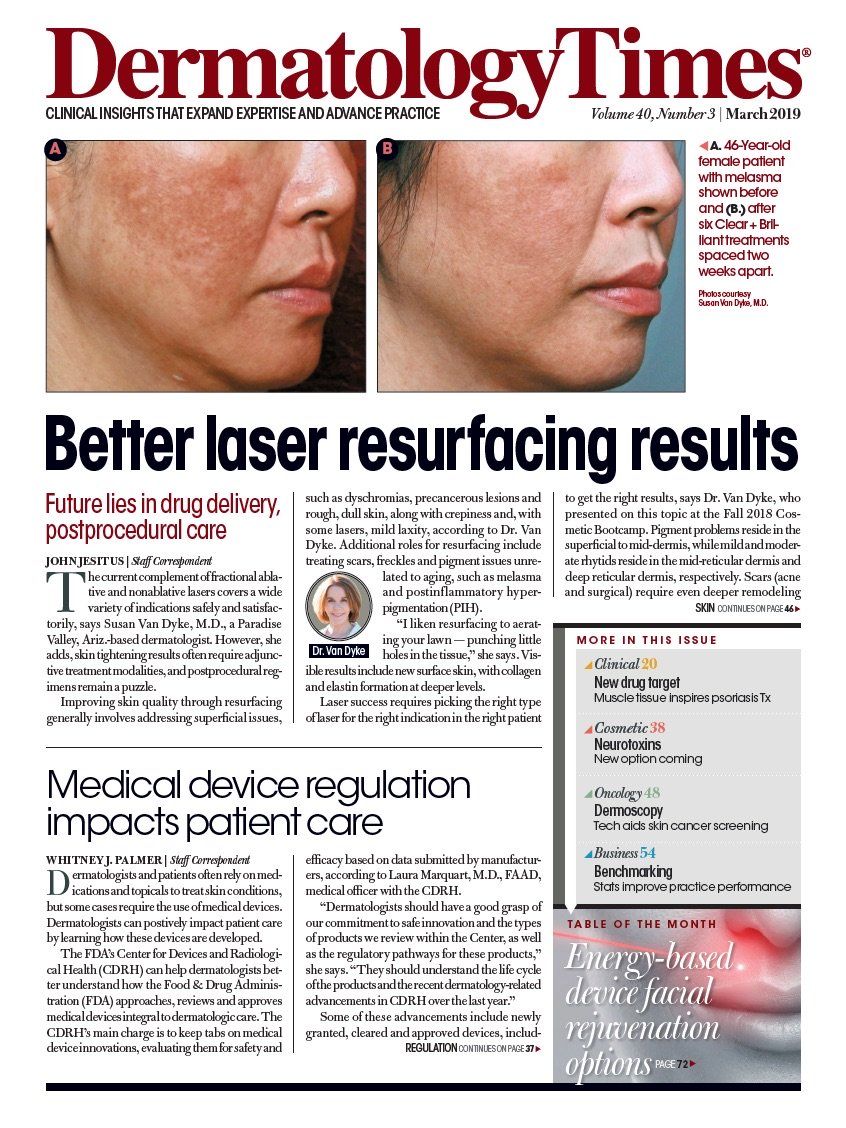
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
FDA oversights for dermatology devices
Author(s):
Learn how the Food and Drug Administration (FDA) plays a critical role in the oversight of many medical devices used commonly in dermatology.
Dermatology is a specialty that employs a wide range of medical devices that range from cosmetic applications to medical dermatology. (lakirr - stock.adobe.com)

Dr. Steve Xu
The Food and Drug Administration (FDA) plays a critical role in the oversight of many medical devices used commonly in dermatology that range from high-risk to low-risk products. Within the FDA, the Center for Devices and Radiological Health (CDRH) is the responsible Center for the regulation of medical devices. Overall, the FDA’s CDRH has grouped 1,700 different generic types of devices into 16 medical specialties referred to as panels. Dermatological devices are often (but not always) regulated under the General and Plastic Surgery panel.
Ultimately, the FDA regulates medical devices by categories of risk. The first is the determination of whether a product is a medical device or not. There is a long legal definition to what constitutes a device. But, in summary, a product is considered a medical device if the intended use is to diagnosis, cure, mitigate, treat or prevent disease in man or animals. Importantly, a medical device achieves its primary intended purpose through non-chemical action within or on the body of man. An example of a device used in dermatology that is not considered a medical device are new and emerging UV sensors. By simply reporting external exposure of UV exposure, these sensors do not meet the FDA’s definition of a medical device and can be sold to consumers directly without FDA clearance.
Class I medical devices are considered the lowest risk devices and subject largely to only general controls. These general controls include requirements for the company to follow appropriate labeling rules, and good manufacturing practices. The majority of Class I devices are exempt from premarket notification of the FDA prior to market entry. Bandages, examination gloves, and most handheld surgical instruments (e.g. punch biopsy tools) fall in this category.
Class II devices are considered moderate risk and represents the broadest range of medical devices. Typically, Class II devices undergo clearance by the FDA through the 510(k) pathway where a new medical device can cite a predicate (already approved) device as substantially equivalent. Often, no clinical data is needed to obtain this clearance. There are several prescription moisturizers that are actually regulated as medical devices cleared through the 510(k) pathway.1 The manufacturers have argued that these moisturizers provide benefit via non-chemical means in order to obtain FDA clearance as a medical device rather than a drug. Certain fractional non-ablative lasers also obtain 510(k) clearance as a Class II medical device. Narrow band phototherapy systems are another example of Class II medical devices cleared via the 510(k) pathway.
Class III medical devices pose the highest risk. These devices usually support or sustain life, are implanted, or present a significant potential risk of illness or injury. These devices must follow the strictest regulatory pathway known as the pre-market approval pathway. The device typically requires clinical data demonstrating safety and efficacy prior to FDA approval. The most well-known examples of Class III devices in dermatology are soft tissue fillers.2
Dermatology is a specialty that employs a wide range of medical devices that range from cosmetic applications to medical dermatology. The FDA plays a critical role in the regulation, approval and post-marketing surveillance of the safety and efficacy of these devices. Practicing dermatologists should be made aware of the ability to submit adverse event reports related to medical devices to MedWatch, the FDA’s Safety and Information and Adverse Reporting Program.3 A MedWatcher mobile app4 is now also available. By reporting adverse events directly to the FDA including near misses, dermatologists can play an important role in surveillance of emerging public safety issues related to medical devices in our field. Reporting adverse events to the manufacturer directly is also another option-by law, they are required to forward such reports to the FDA. My suggestion is to do both. Â
References
1. Draelos ZD. An evaluation of prescription device moisturizers. J Cosmet Dermatol. 2009;8(1):40
2. Lohman ME, Ghobadi CW, Xu S. Device Safety Implications of the Clinical Data Leading to US Food and Drug Administration Approval of Soft-Tissue Fillers: A Systematic Review. JAMA Facial Plast Surg. 2017;19(5):421-429.
3. FDA. MedWatch Voluntary Reporting Form. https://www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=professional.reporting1 Accessed February 2019.
4. FDA. MedWatcher Mobile App. https://www.fda.gov/MedicalDevices/Safety/ReportaProblem/ucm385880.htm Accessed February 2019.