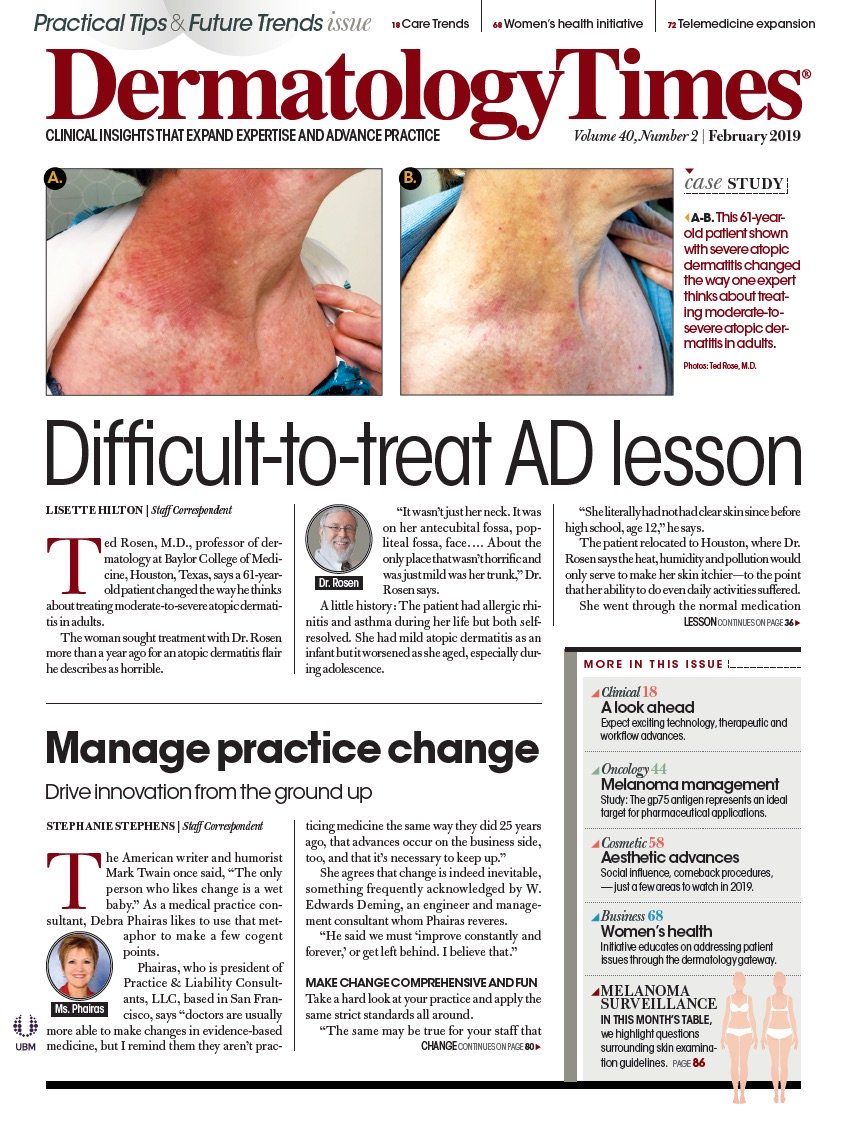
- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Non-melanoma skin cancer trial proceeds
Author(s):
The FDA has approved STP705, an siRNA (small interfering RNA) therapeutic for in situ Squamous Cell Carcinoma Nonmelanoma Skin Cancer (NMSC), to proceed with phase two clinical trials.
The FDA has approved STP705, an siRNA (small interfering RNA) therapeutic for in situ Squamous Cell Carcinoma Nonmelanoma Skin Cancer (NMSC), to proceed with phase two clinical trials.
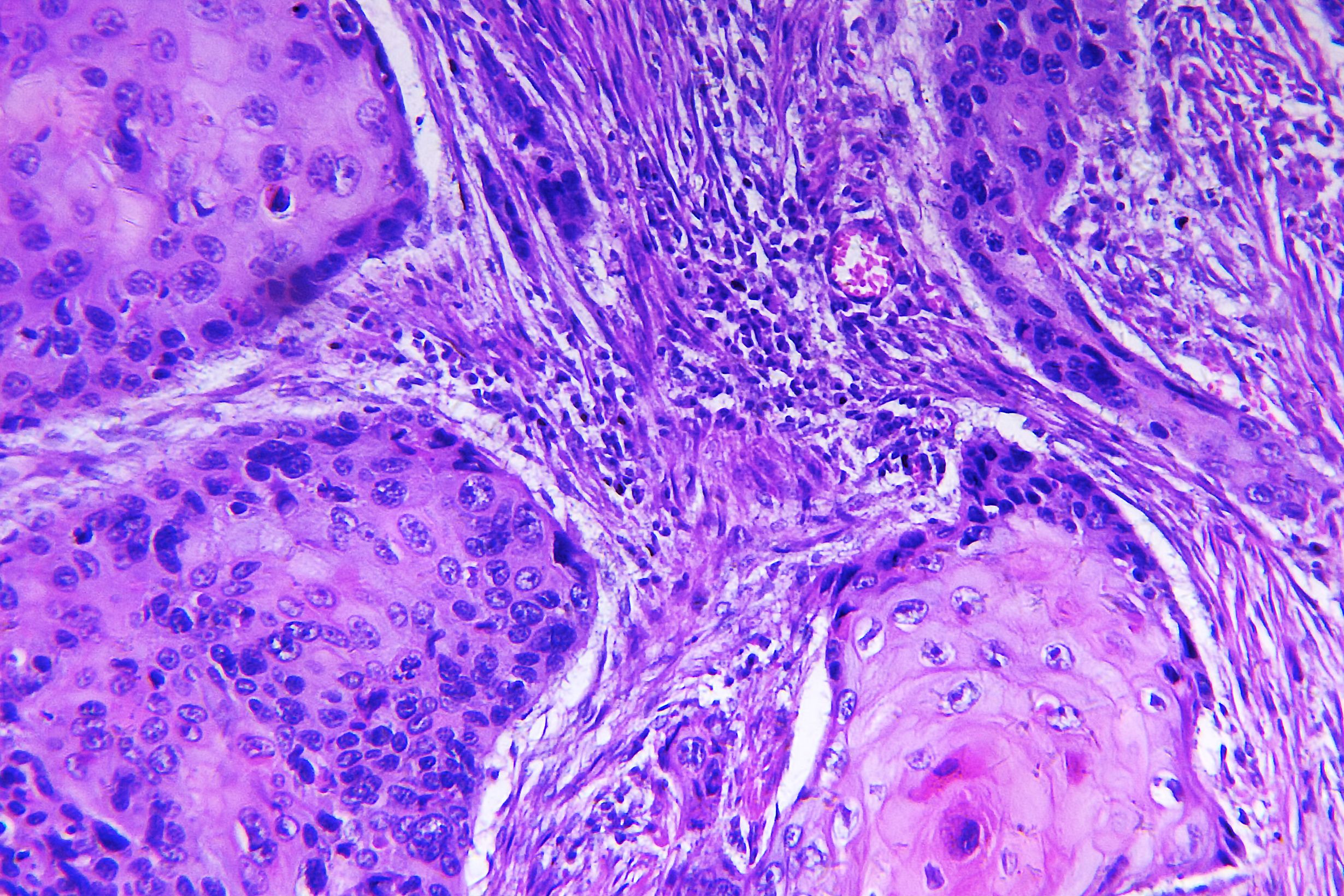
Sirnaomics, Inc., announced in December that it received FDA approval to proceed with phase two clinical trials for
STP705, an siRNA (small interfering RNA) therapeutic for in situ Squamous Cell Carcinoma Nonmelanoma Skin Cancer (NMSC). Trials are expected to start in the first quarter of 2019.
STP705 works by inhibiting the expression of TGF-β1 and COX-2, enzymes that contribute to epithelial cell proliferation and the regulation, development and progression of NMSC.
“FDA clearance to proceed with the NMSC trial represents an important step forward in demonstrating the broader clinical utility of our siRNA platform,” said Sirnaomics’ founder and CEO Patrick Y. Lu, Ph.D. “Building on our technology’s potential against fibrotic disease, the drug target selection and tumor targeting delivery capabilities of our platform will help demonstrate the broader therapeutic potential of STP705 as a therapeutic candidate against cancer.”
The phase two trial will be led by Brian Berman, M.D., Ph.D., of the University of Miami Miller School of Medicine. “There is a real clinical need for non-invasive, targeted therapy for in situ squamous cell carcinoma,” Dr. Berman said in a news release.
Sirnaomics specializes in the discovery and development of RNAi therapeutics against cancer and fibrotic diseases.