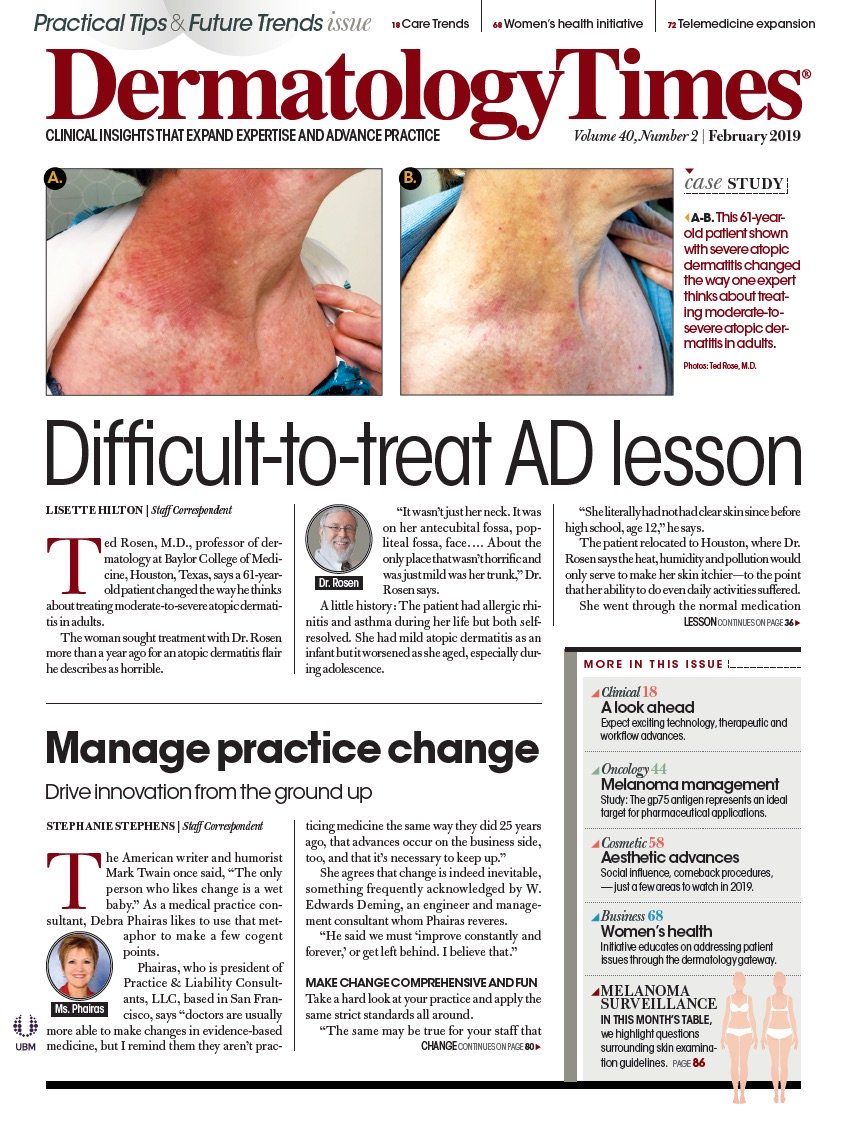
- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Topical for molluscum contagiosum performs well in phase 3 trials
Author(s):
Novel therapeutic recently completed phase three CAMP-1 and CAMP-2 pivotal trials, demonstrating statistically significant improvements in patients with molluscum contagiosum.
VP-102 recently completed phase three CAMP-1 and CAMP-2 pivotal trials, demonstrating statistically significant improvements in patients with molluscum contagiosum. (Lukassek - stock.adobe.com)
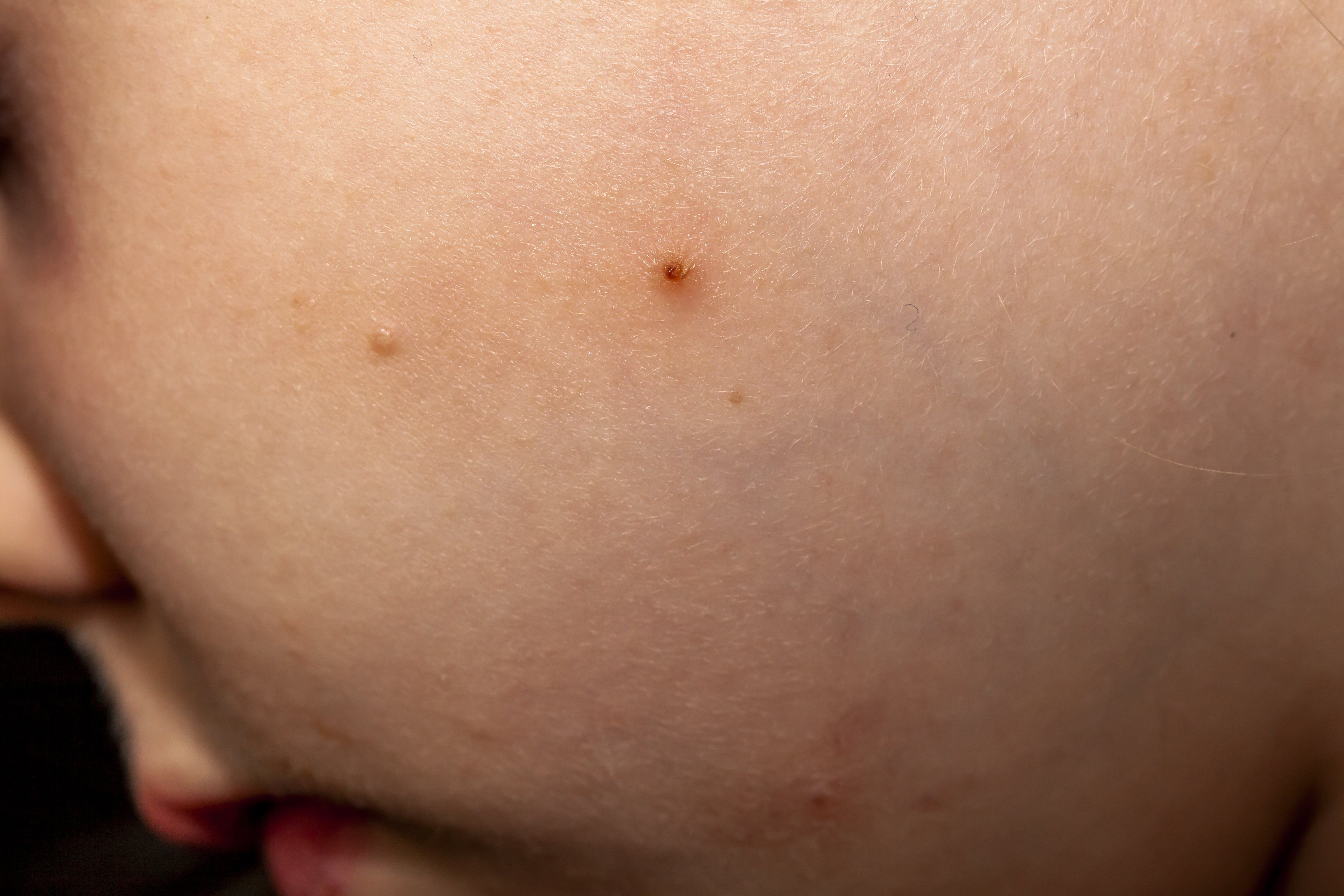
Verrica Pharmaceuticals announced in January that it’s novel therapeutic, VP-102, recently completed phase three CAMP-1 and CAMP-2 pivotal trials, demonstrating statistically significant improvements in patients with molluscum contagiosum, a chronic virus marked by pearl-like bumps on the skin primarily in children.
Currently, there are no FDA-approved treatments for the condition, but Verrica has been sponsoring clinical trials for VP-102, it’s own novel topical solution consisting of 0.7% cantharidin.
“In each trial, VP-102 exhibited a clinically and statistically significant proportion of subjects demonstrating complete clearance of all treatable molluscum lesions versus placebo. VP-102 was well-tolerated in both trials, with no serious adverse events reported in VP-102 treated subjects,” the company announced in a statement.
“We believe the efficacy and safety profiles of VP-102 observed in these two trials provide a strong foundation for our U.S. NDA which we intend to submit in the second half of this year,” said Ted White, Verrica’s president and chief executive officer.
CAMP-1 and 2 are two randomized, doubleblind, multicenter, placebo-controlled trials conducted at 31 centers in the United States. In total, the studies included patients two years old or older. The patients were treated once every 21 days for up to four applications for 12 weeks.
In CAMP-1, 46% of patients treated with VP-102 achieved complete clearance of all treatable molluscum lesions at day 84 versus 18% of placebo groups. In CAMP-2, 54% of treated patients achieve complete clearance as compared to 13% in the placebo group. And, also by the end of the trail, treated patients achieved a 69% and 83% mean reduction, respectively, in the number of molluscum lesions as compared to 20% and 19% for subjects on placebo.
Molluscum contagiosum affects six million people in the United States.