- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
What's new in melanoma treatment?
Author(s):
Innovative melanoma therapeutics are on the horizon. This pipeline report highlights some of the new drugs currently in phase 2, phase 3 or recently approved for the treatment of melanoma.
Know these innovative melanoma therapeutics on the horizon. (Christoph Burgstedt - stock.adobe.com)
This pipeline report presents insights into drugs currently in phase 2, phase 3 or recently approved for the treatment of melanoma.
In 2018, an estimated 96,480 patients in the United States were diagnosed with melanoma, and about 7,230 patients died from the disease.1,2 Drug development within melanoma has focused on cutaneous melanoma therapeutics and later stages of disease when surgery is not an option.
Melanoma has historically been a disease that is difficult to treat with pharmacotherapy and drug development made limited progress. In recent
years, developments in molecular biology, have led to an increased understanding of the molecular heterogeneity of melanoma that has resulted in introducing new insights into the role of oncogenes, immune checkpoints, and signaling pathways which has accelerated the discovery rate of therapeutic targets and their associated novel drugs. Breakthrough drug approvals in recent years, checkpoint inhibitors and MEK/BRAF combination therapies have prolonged survival and changed the treatment landscape.
Even with the impact of these breakthrough therapies, unmet needs still remain for safer and more effective therapies. The greatest promise in addressing these needs may come in the form of combining novel therapeutics with currently marketed therapies to provide effective treatment and improve patient survival.
Know these innovative melanoma therapeutics on the horizon.
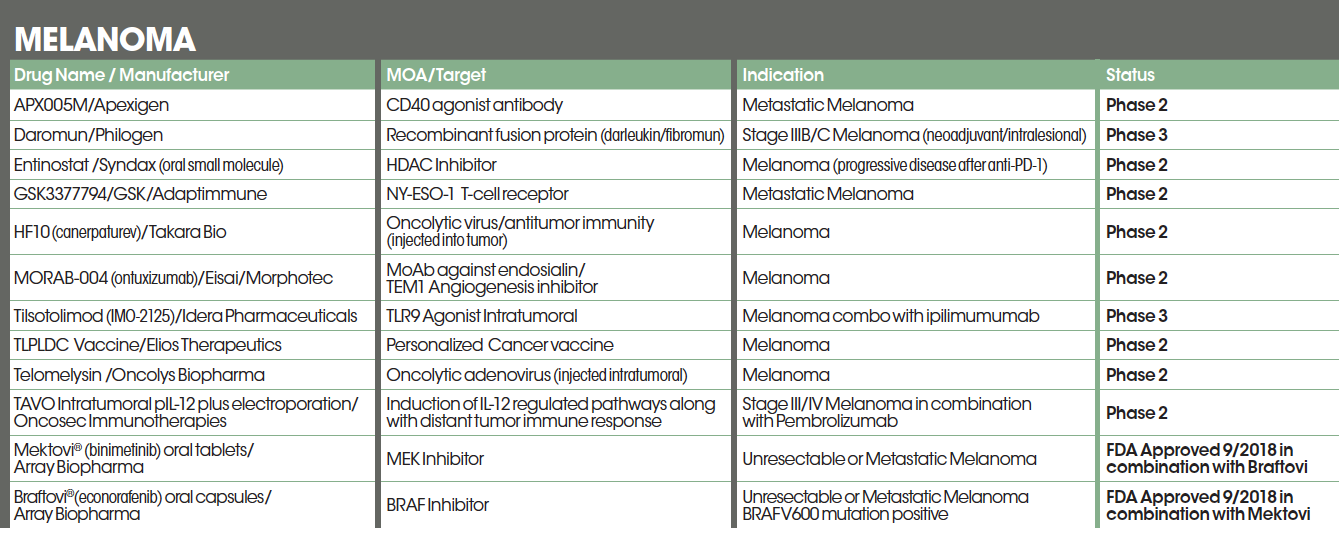
APX005M BY APEXIGEN
APX005M is a humanized monoclonal antibody and a potent CD40 agonist designed to reverse the systemic immune suppression. CD40 is a co-stimulatory receptor that is essential for activating both innate and adaptive immune systems. APX005M is administered by intratumoral injection and has the potential to be used as a single agent and in combination with other immune-oncology agents, targeted therapies and vaccines.3 A phase 1/2 dose escalation and cohort expansion, safety and tolerability study of intratumoral APX005M in combination with systemic pembrolizumab in 41 patients with metastatic melanoma is scheduled to be completed in June of 2020.4
DAROMUN BY PHILOGEN
Daromun is a combination of Darleukin, a human vascular targeting monoclonal antibody (L19) fused to interleukin-2 (IL-2), and Fibromun, the L19 antibody linked to tumor necrosis factor (TNF). Daromun is being developed as a neoadjuvant intralesional treatment for stage IIIB and C melanoma.
Philogen received an Orphan Drug designation from the FDA in 2017. Daromun would be the first neoadjuvant therapy aimed at blocking or delaying progression to stage IV melanoma. In a phase 2 study of intralesional treatment with Daromun in stage III/IVA melanoma patients (EudraCT No. 2012-001991-13) efficacy was demonstrated in terms of complete and partial response of the injected metastases, as well as a systemic immune response in non-injected lesions that resulted in shrinking the lesions or in some cases disappearing. In addition, a decelerated progression to stage IV melanoma of treated patients with respect to historical controls was recorded.
A randomized, controlled phase 3 registration trial of intralesional application of Daromun as a neoadjuvant, followed by surgery and compared to surgery alone is presently ongoing in Europe and USA with a projected enrollment of 248 patients with fully resectable stage IIIB/C melanoma. The primary endpoint is recurrence-free survival, with overall survival as key secondary endpoint.5,6
ENTINOSTAT BY SYNDAX
Entinostat (ENT) is an oral class-1 selective histone deacetylase inhibitor that leads to downregulation of immunosuppressive cells in the tumor microenvironment. In preclinical studies, entinostat demonstrated synergy with anti-PD1 inhibitors.7
In a phase 2 open-label study evaluating ENT plus Pembro in patients with recurrent or metastatic melanoma and prior progression on or after
Anti-PD1 therapy, 53 patients were treated. The results demonstrated 10 confirmed responses (19% ORR) with 1 CR and 9 PRs. The responses were durable with a median duration of 13 months. An additional nine patients had stable disease for greater than six months.8,9 ENT plus Pembro is a promising combination therapy for patients with progressing melanoma on prior PD-1 blockade and both PD-1 and CTLA-4 blockade, a group with limited treatment options.
GSK3377794 (GSK794) BY GLAXOSMITHKLINE AND ADAPTIMMUNE
GSK794 is an engineered T-cell therapy, for which a patient’s own cells have been genetically modified to express a T-cell receptor (TCR) recognizing with high affinity the tumor-specific antigen, NY-ESO. When the modified cells are re-infused into the patient, they recognize and kill tumor cells that express the NY-ESO antigen.
NY-ESO is expressed at various levels across different tumors and appears to be expressed at high levels in defined sub-types of melanoma. GSK794 is currently being studied in seven phase I/II studies assessing its effectiveness as a treatment for non-small cell lung cancer, metastatic melanoma, ovarian cancer, multiple myeloma, synovial sarcoma, and myxoid round cell liposarcoma. It has been granted PRIME designation by the European Medicines Agency and Breakthrough Therapy designation by the U.S. Food and Drug Administration, underlining the significant potential benefits it can provide.10
HF10 (CANERPATUREV) BY TAKARA BIO
Canerpaturev (C-REV) is an attenuated strain of the herpes simplex virus type 1 (HSV-1) that demonstrates anti-tumor activity when locally injected into a tumor. This type of virus is called “oncolytic virus.” Oncolytic viruses do not replicate inside of normal cells, but selectively replicate in tumor cells and break them down without excessively damaging normal cells.11
A phase 2 study of combination with HF10, a replication-copetent HSV-1 oncolytic virus, and Ipilimumab in Japanese patients with stage IIIB m IIIC, or IV unresectable or metastatic melanoma was completed. The data showed a favorable benefit/risk profile and promising antitumor activity.12
MORAB-004 (ONTUXIZUMAB) BY EISAI, MORPHOTEC
MORAB-004 (ontuxizumab) is a potential first-in-class humanized monoclonal antibody against endosialin/TEM1(tumor endothelial marker), which plays an important role in tumor stromal cell function, angiogenesis, tumor growth, and metastasis.13,15
In a global phase 2 , open-label, dose selection, proof-of-concept study to assess progression free survival (PFS) in subjects with metastatic melanoma, 76 patients received at least one dose of ontuxizumab. The PFS at 24 weeks was 11.4% for all patients (13.5% for 2mg/kg and 8.9% for 4mg/kg). One patient receiving 4 mg/kg had a partial response and twenty-seven of 66 response evaluable patients (40.9%) had stable disease. The median overall survival was 31 weeks. Ontuxizumab at both doses was well tolerated.
TILSOTOLIMOD (IMO-2125)BY IDERA
Tilsotolimod is synthetic oligonucleotide agonist of toll-like receptor 9 designed to turn on or activate the immune system by improving antigen presentation of dendritic cells and macrophages with subsequent proliferation of antigen-specific cytotoxic T lymphocytes. Tilsotolimod is injected intratumorally to stimulate an immune response within the tumor microenvironment to enable stronger anti-tumor activity from check point inhibitors such as anti-PD-1 and CTLA-4 inhibitors.16 An analysis of 21 patients from the ILLUMINATE-204 study was presented at the ESMO Conference in 2018. Patients were treated with tilsotolimod plus ipilimumab. The overall response rate was 38% which included two complete responses and six partial responses. An additional seven patients had stable disease and six had progressive disease.
Tilsotolimo plus ipilimumab revives the immune response in anti-PD-1 resistant tumors. Efficacy was observed in injected and uninjected distant lesions, demonstrating an abscopal effect. The combination had durable responses. The combination was well tolerated with only six subjects experiencing immune-related toxicities.17,18 There is an ongoing randomized phase 3 study comparing tilsotolimod plus ipilimumab to ipilimumab alone in the anti-PD-1 refractory melanoma population.19
TLPDC VACCINE BY ELIOS THERAPEUTICS
Tumor lysate particle loaded dendritic cells (TLPLDC) is an autologous therapeutic cancer vaccine that is made from the patient’s own cells and is designed to stimulate the immune system to recognize tumor cells and fight the patient’s specific cancer. The vaccine is made from the patient’s own tumor tissue (1mg collected during surgery) and 120 ml of the patient’s blood. It takes only 48 hours to produce the vaccine. Patients receive the initial vaccine and then three monthly follow-up vaccine inoculations.
The TLPLDC vaccine is scalable.20 Based on data from a Phase 1/ 2 trial, the vaccine is safe, with primarily grade 0–2 toxicities, and nearly 40% clinical benefit rate in varied tumors, thus warranting further study. An interim analysis of a prospective, randomized, double-blind, placebo-controlled, phase 2b trial of TLPLDC vaccine to prevent recurrence in resected stage III or IV melanoma patients was presented at the 2018 ASCO Annual Meeting. In the study, 120 participants were randomized 2:1 to receive either TLPLDC or placebo to prevent recurrence.
The analysis showed a meaningful 32% reduction in the relative risk of recurrence with a median follow-up of 12.6 months. Overall TLPLDC was safe and well-tolerated; only 33% of participants experienced an adverse event and 98% were grade 1 and 2 events. These data provide a strong rationale for developing a phase 3 study.21
TELOMELYSIN (OBP-301) BY ONCOLYS BIOPHARMA
Telomelysin is a gene-modified oncolytic adenovirus which selectively replicates in cancer cells by introducing a human telomerase reverse transcriptase (hTERT) promotor. Oncolytic adenovirus has much potential for cancer immunotherapy because its viral replication is highly immunogenic, and oncolysis induced by such virus releases tumor antigen and provides co-stimulatory danger signals.
OBP-301 has demonstrated regression in the injected and noninjected melanoma lesions (abscopal effect).22 An open-label, multi-center phase 2a study to evaluate efficacy and immunological response of intratumoral/intralesional oncolytic virus (OBP-301) in unresectable/unresected metastatic melanoma is active and seeking to enroll 50 patients.23
TAVO (TAVOKINOGENE TELSEPLASMID) INTRATUMORAL PIL-12 PLUS ELECTROPORATION BY ONCOSEC IMMUNOTHERAPIES
OncoSec’s immunotherapy platform, Intratumoral IL-12, focuses on the delivery of DNA-based interleukin-12 (IL-12), a naturally occurring protein with immune-stimulating functions. The treatment is designed to produce a controlled, localized expression of IL-12 in the tumor microenvironment, which, in turn, enables the immune system to target and attack tumors throughout the body.
The ImmunoPulse device is used to supply a sequence of short-duration electrical pulses (electroporation) to the tumor which increases the permeability of the cell membrane and facilitates the uptake of the IL-12.24
A multicenter phase 2, open-label trial of intratumoral pIL-12 plus electroporation in combination with intravenous pembrolizumab in patients with stage III/IV melanoma who are progressing on either pembrolizumab or nivolumab treatment is currently open and accruing patients. The primary outcome measure is overall response rate with secondary outcomes of progression free survival, overall survival, and duration of response.25
BRAFTOVI ( ENCORAFENIB) + MEKTOVI (BINIMETINIB) COMBINATION BY ARRAY BIOPHARMA
Braftovi (encorafenib) is indicated in combination with Mektovi (binimetinib) for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test. Encorafenib and binimetanib target two different kinases in the RAS/RAF/MEK/ERK pathway. The co-administration of these drugs resulted in a synergistic anti-proliferative activity in vitro in BRAF mutation-positive cell lines and human xenograft studies.26,27
In a two-part, randomized, open label, multi-center, parallel group, phase 3 study comparing the efficacy and safety of LGX818 (econorafenib) plus MEK 162 (binimetinib) to vemurafenib and LGX818 monotherapy in patients with locally advanced unresectable or metastatic melanoma with BRAF V600 mutations, a total of 577 patients were randomized; 192 to the econorafenib+binimetinib arm, 194 to the econorafenib arm, and 191 to the venurafenib arm. Econorafenib + binimetinib demonstrated a statistically significant improvement in PFS compared to vemurafenib (median PFS 10 months vs 2 months p<0.0001). The overall response rate was 63% vs 40% and the duration of response 16.6 months vs 12.3 months.27
References:
The data for this article were compiled from the Pharmaceutical Research and Manufacturers of America 2018 Medicines in Development for Cancer Report; NIH www.clinicaltrials.gov; corporate websites, and Pubmed.
1. NCCN Clinical Practice Guidelines: Cutaneous Melanoma; Version 2.2019, March 2019.
2. American Cancer Society. Cancer Facts & Figures 2019. Atlanta: American Cancer Society;2019
3. Apexigen.com
4. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT02706353. APX005M in Combination With Systemic Pembrolizumab in Patients With Metastatic Melanoma. Available: https://clinicaltrials.gov/ct2/show/NCT02706353. Updated May 9, 2019.
5. Philogen.com
6. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT03567889. Efficacy of Daromun Neoadjuvant Intratumoral Treatment in Clinical Stage IIIB/C Melanoma Patients (Neo-DREAM). Available: https://clinicaltrials.gov/ct2/show/NCT03567889. Updated March 21, 2019.
7. Syndax.com
8. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT02437136. Ph1b/2 Dose-Escalation Study of Entinostat With Pembrolizumab in NSCLC With Expansion Cohorts in NSCLC, Melanoma, and Colorectal Cancer. Available: https://clinicaltrials.gov/ct2/show/NCT02437136. Updated November 28, 2018.
9. Sullivan R, et al. Efficacy and safety of entinostat (ENT) and pembrolizumab (PEMBRO) in pati3nts with melanoma previously treated with anti-PD-1 therapy. 2019 American Association for Cancer Research Annual Meeting, Atlanta, GA.
10. GSK.com
11. Takara-bio.com
12. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT03153085. A Study of Combination With TBI-1401(HF10) and Ipilimumab in Japanese Patients With Unresectable or Metastatic Melanoma. Available: https://clinicaltrials.gov/ct2/show/NCT03153085. Updated January 24, 2019.
13. Eisai.com
14. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT01335009. Efficacy Study of Pharmacokinetic(PK)/Pharmacodynamic(PD) Relationship of Monotherapy MORAb-004 in Metastatic Melanoma. Available: https://clinicaltrials.gov/ct2/show/NCT01335009. Updated April 22, 2019.
15. D’Angelo SP, et al. A phase 2 study of ontuxizumab, a monoclonal antibody targeting endosialin, in metastatic Melanoma. Investigational New Drugs 2018.36(1):103-113.
16. Iderapharma.com
17. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT02644967. A Study to Assess the Safety and Efficacy of Intratumoral IMO-2125 in Combination With Ipilimumab or Pembrolizumab in Patients With Metastatic Melanoma (ILLUMINATE-204). Available: https://clinicaltrials.gov/ct2/show/NCT02644967. Updated April 25, 2019.
18. Diab A, et al. The safety and efficacy of intratumoral injection of the TLR9 agonist tilsotolimod (IMO-2125) in combination with ipilimumab in patients with PD-1 inhibitor refractory metastatic melanoma: An analysis of efficacy in injected and uninjected lesions. Presented at ESMO Congress, October 2018.
19. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT03445533. A Study of IMO-2125 in Combination With Ipilimumab Versus Ipilimumab Alone in Subjects With Anti-PD-1 Refractory Melanoma (ILLUMINATE-301) (ILLUMINATE-301). Available: https://clinicaltrials.gov/ct2/show/NCT03445533. Updated May 10, 2019.
20. Eliostherapeutics.com
21. Myers j. Interim analysis of a prospective , randomized, double-blind, placebo-controlled, Phase 2b trial o TLPLDC vaccine to prevent recurrence in resected Stage III or IV melanoma patients. 2018 ASCO Annual Meeting , Poster #352 Melanoma/Skin Cancers Poster Session, June 4, 2018.
22. Oncolys.com
23. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT03190824. Evaluate Efficacy, Immunological Response of Intratumoral/Intralesional Oncolytic Virus (OBP-301) in Metastatic Melanoma. Available: https://clinicaltrials.gov/ct2/show/NCT03190824. Updated April 16, 2019.
24. oncosec.com
25. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT03132675. Tavo and Pembrolizumab in Patients With Stage III/IV Melanoma Progressing on Pembrolizumab or Nivolumab Treatment (PISCES). Available: https://clinicaltrials.gov/ct2/show/NCT03132675. Updated April 5, 2019.
26. Arraybiopharma.com; braftovimektovi.com
27. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). NCT01909453. Study Comparing Combination of LGX818 Plus MEK162 Versus Vemurafenib and LGX818 Monotherapy in BRAF Mutant Melanoma (COLUMBUS). Available: https://clinicaltrials.gov/ct2/show/NCT01909453. Updated April 26, 2019.






