
- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Shanna Miranti, MPAS, PA-C, Shares Insights on Newly Approved VP-102 for Molluscum Contagiosum
Author(s):
Providers have been eagerly awaiting the approval of VP-102, as molluscum contagiosum accounts for approximately 1% of all diagnosed skin conditions.
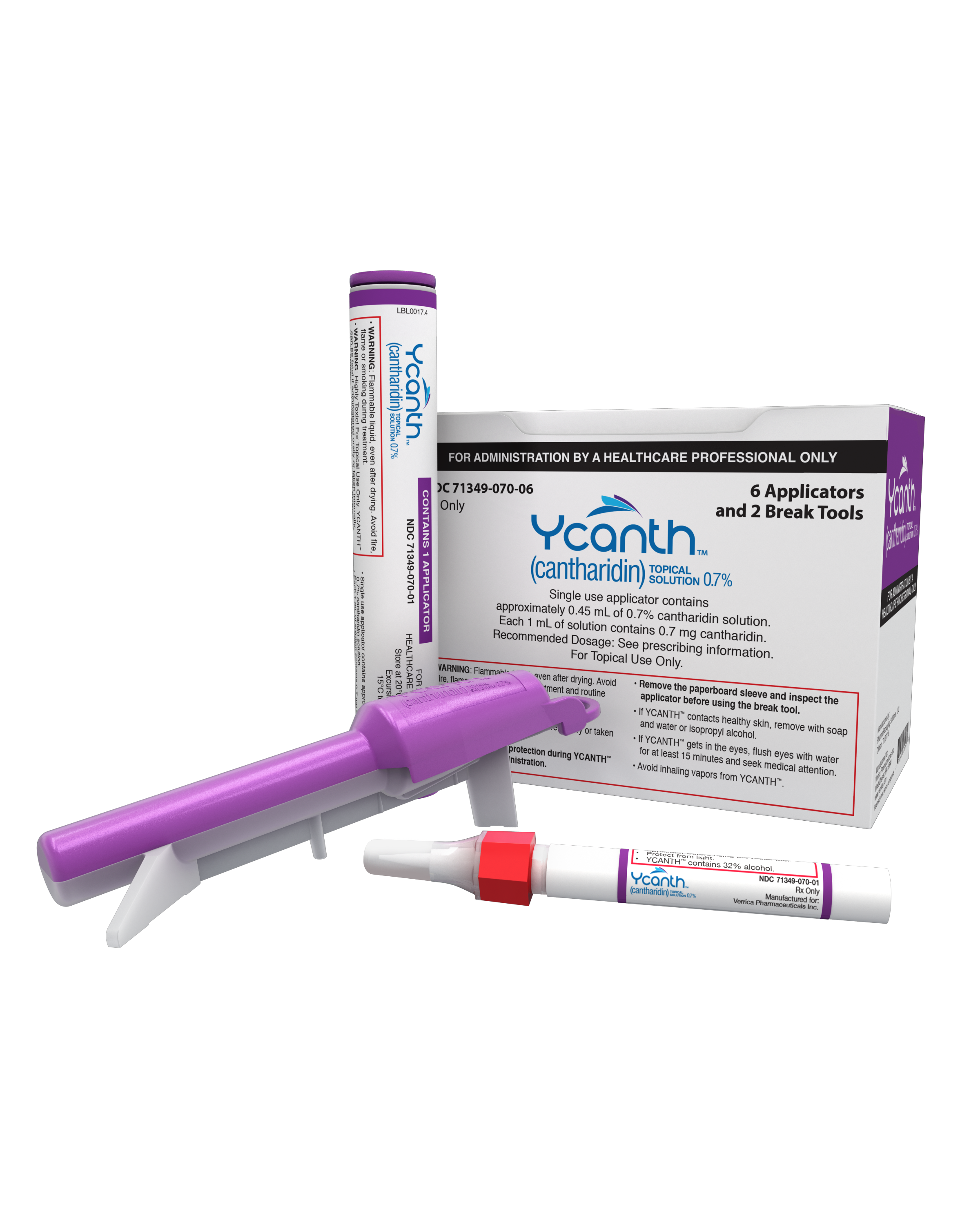
Verrica Pharmaceuticals’ VP-102 (Ycanth) was approved by the US Food and Drug Administration (FDA) for the treatment of molluscum contagiosum on July 21, 2023. VP-102 is a drug-device combination that contains a Current Good Manufacturing Process–controlled formulation of cantharidin 0.7% w/v and gentian, a surgical dye meant to clearly mark treated lesions. VP-102 is the first FDA-approved drug for the treatment of molluscum contagiosum and offers dermatology providers and patients a precise, in-office treatment.1
Shanna Miranti, MPAS, PA-C

Providers have been eagerly awaiting the approval of VP-102, as molluscum contagiosum accounts for approximately 1% of all diagnosed skin conditions and is 1 of the 50 most common skin conditions. Molluscum contagiosum most commonly affects pediatric patients, beginning around age 6 years.2
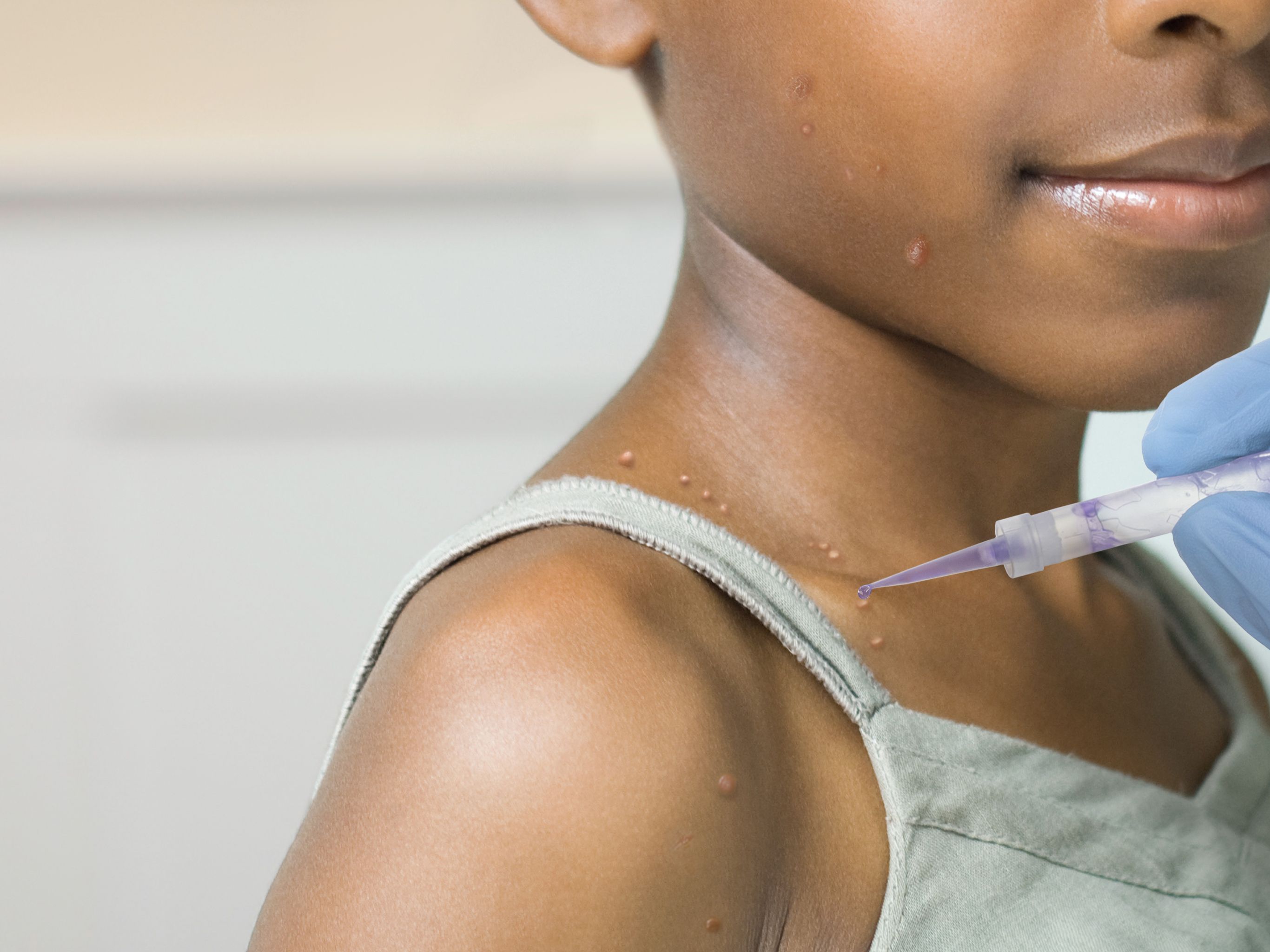
With VP-102, providers can apply a precise amount of cantharidin 0.7% to each individual molluscum lesion, while also marking each treated lesion with a violet dye to further prevent missed lesions or duplicate dosing.
Shanna Miranti, MPAS, PA-C, a board-certified physician assistant at Riverchase Dermatology in Naples, Florida, specializes in adult and pediatric dermatology and treats molluscum contagiosum frequently at her practice. Dermatology Times spoke with Miranti to discuss VP-102’s approval and how previously there have not been ideal standards of care.
Q: Can you please explain the significance of VP-102 as a drug-device that precisely applies cantharidin 0.7% and the surgical dye gentian?
Miranti: Molluscum contagiosum is a very common associated viral condition that affects 6 million Americans, the majority of those being children, teens, and young adults. As of very recently, we have never had an FDA-approved treatment option for this very persistent, very stubborn skin condition. Until now, we finally have our very first FDA-approved product, Ycanth, which was known as VP-102, which is a drug-device combination product that applies a very small, precise amount of cantharidin and a surgical dye and is applied in office to the patient’s lesions. We can very specifically treat the individual raised bumps. There is a purple-stained dye that is contained in the medication combination, as well, that allows us to see the precise application location, so we know exactly what bumps have been treated and what bumps have not. This will truly start to help those providers who see molluscum—and there are a lot of us out there—gain consistency in our treatments. Because up until now, we’ve had no consistency.
Q: What has been the previous standard of care for treating molluscum lesions in pediatric patients?
Miranti: Previously, the standard of care in our pediatric patient population was a little bit all over the place because we had no specific guidelines. We had no specific FDA-approved product. Many of us were using compounded cantharidin, which in theory is a great option. The problem is not all compounds are created equal. There’s a lot of variability; there are a lot of variations among compounding companies. Sometimes the products that you get are not the same as the lot that you ordered last month, even if you’re ordering from the exact same manufacturer. Cantharidin is a product that has been around since the 1950s. It was originally introduced in medicine to start to treat molluscum, even very early on, but it was pulled from the market in the 1960s because of lack of efficacy data submitted to the FDA. So even though it’s been a theorized medication, there was never an FDA-approved option for cantharidin. We’ve been using compounded cantharidin in our little brown bottle. For those of us who have been treating patients for years with cantharidin—or, as we call it “beetle juice”—we have been grabbing our little brown bottle from the cabinet and treating our patients. Unfortunately, there’s not always consistency. Sometimes patients will receive a larger bolus of medication; sometimes certain lesions are missed. We really want all dermatology providers across the country to have a consistent product that’s reliable, readily available, and easy to use.
Image courtesy of Verrica Pharmaceuticals
Q: Why is compounded cantharidin not the best treatment selection for molluscum?
Miranti: In early June 2023, the FDA came out with a pretty strongly worded consumer warning against using compounded cantharidin. Because of the lack of consistency across manufacturers, both in the country or manufacturers in Canada, the FDA was warning providers and patients that there was no FDA-approved option yet. The FDA warned that patients needed to be very careful and cautious about products that were commercially available on Amazon or over the counter or through mail-order websites that have not actually proven efficacy to the FDA. There are a number of potential concerns and potential adverse effects that can occur from products that are not FDA approved. Because of the FDA’s strong warning, we as providers really need to pay attention to that. We need to start utilizing the options that are truly FDA approved and have been proven both safe and efficacious for our patients.
Q: How do you hope to see VP-102 change the way molluscum contagiosum is treated in terms of efficacy and ease of use?
Miranti: I do believe we are going to have a new gold standard. We will have a new standard way of treating molluscum contagiosum when it comes into our offices. We will have the Ycanth product available and in our offices to start treating our patients as soon as it is available. This is a product that will deliver a very precise amount of product to each individual molluscum and will be provider administered, so we will do these treatments in office. This is not a take-home product for patients to use on their own. This will be something applied precisely in the office location.
There will be a couple of small growing pains as we get used to this new model. There will be either a buy-and-bill system or there is an Rx model as well, where we can write a patient a prescription and then have the product sent to our office and have the treatment ready for that patient’s next appointment. We will be able to devise a system that works for our procedures and our policies in the office and hopefully will help optimize workflow. This is a product that’s going to be ready to use and ready to go when our patient is there seeking treatment.
Q: What other options may be available as additional treatments for molluscum contagiosum?
Image courtesy of Verrica Pharmaceuticals
Miranti: For a while, there were 2 options that were in a race to the finish to see who was going to get the first FDA approval. We now know that Ycanth is the clear winner. Berdazimer gel, 10.3% (Novan), is hopefully going to be coming out in 2024. There have been some delays in that product coming to launch,3 but we hope to eventually have a take-home option, as well, which is berdazimer gel in a nitric oxide–releasing solution. It’s going to be 2 products in 2 tubes that will have to be mixed together and precisely applied at home for the patient, so we will need to do some counseling for our patients. Berdazimer is going to be an option that we can write as a prescription when we see these patients if they choose not to treat in office and if they wish to bring a prescription home. But as of right now, that is not FDA approved and is not available for our use just yet, but stay tuned.
Shanna Miranti, MPAS, PA-C, is a founding member of and a board-certified physician assistant at Riverchase Dermatology in Naples, Florida. She is also a Diversity in Dermatology-leader, Achieve program mentor, and industry relations chair, and member of the Florida Academy of Physician Assistants and the Society of Dermatology Physician Assistants.
References
- Verrica Pharmaceuticals announces FDA approval of Ycanth (cantharidin) topical solution as the first FDA approved treatment of pediatric and adult patients with molluscum contagiosum. Verrica Pharmaceuticals. July 21, 2023. Accessed July 21, 2023. https://verrica.com/press_release/verrica-pharmaceuticals-announces-fda-approval-of-ycanth-cantharidin-topical-solution-0-7/
- Eichenfield LF, Kwong P, Gonzalez ME, et al. Safety and efficacy of VP-102 (cantharidin, 0.7% w/v) in molluscum contagiosum by body region: post hoc pooled analyses from two phase III randomized trials. J Clin Aesthet Dermatol. 2021;14(10):42-47.
- Novan enters into agreement to sell substantially all of its assets, including berdazimer gel, 10.3% (SB206), and files for chapter 11 protection. Novan. July 17, 2023. Accessed July 17, 2023. https://novan.com/novan-enters-into-agreement-to-sell-substantially-all-of-its-assets-including-berdazimer-gel-10-3-sb206-and-files-for-chapter-11-protection/
















Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.










2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.





