- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Dermatology Times
Psoriasis Updates: Year Thus Far
Author(s):
This Psoriasis Awareness Month, Dermatology Times is looking back at the treatment and research landscape of 2023.
August is National Psoriasis Awareness month and is a reminder for providers to consider how they treat and manage psoriasis in their patients, learn what’s new in therapy, and possibly reassess their treatment strategies. In 2023 and beyond, patients with psoriasis fortunately have more to look forward to in terms of newly available systemic and topical treatment options, as well as those upcoming in the pipeline.
hriana/AdobeStock
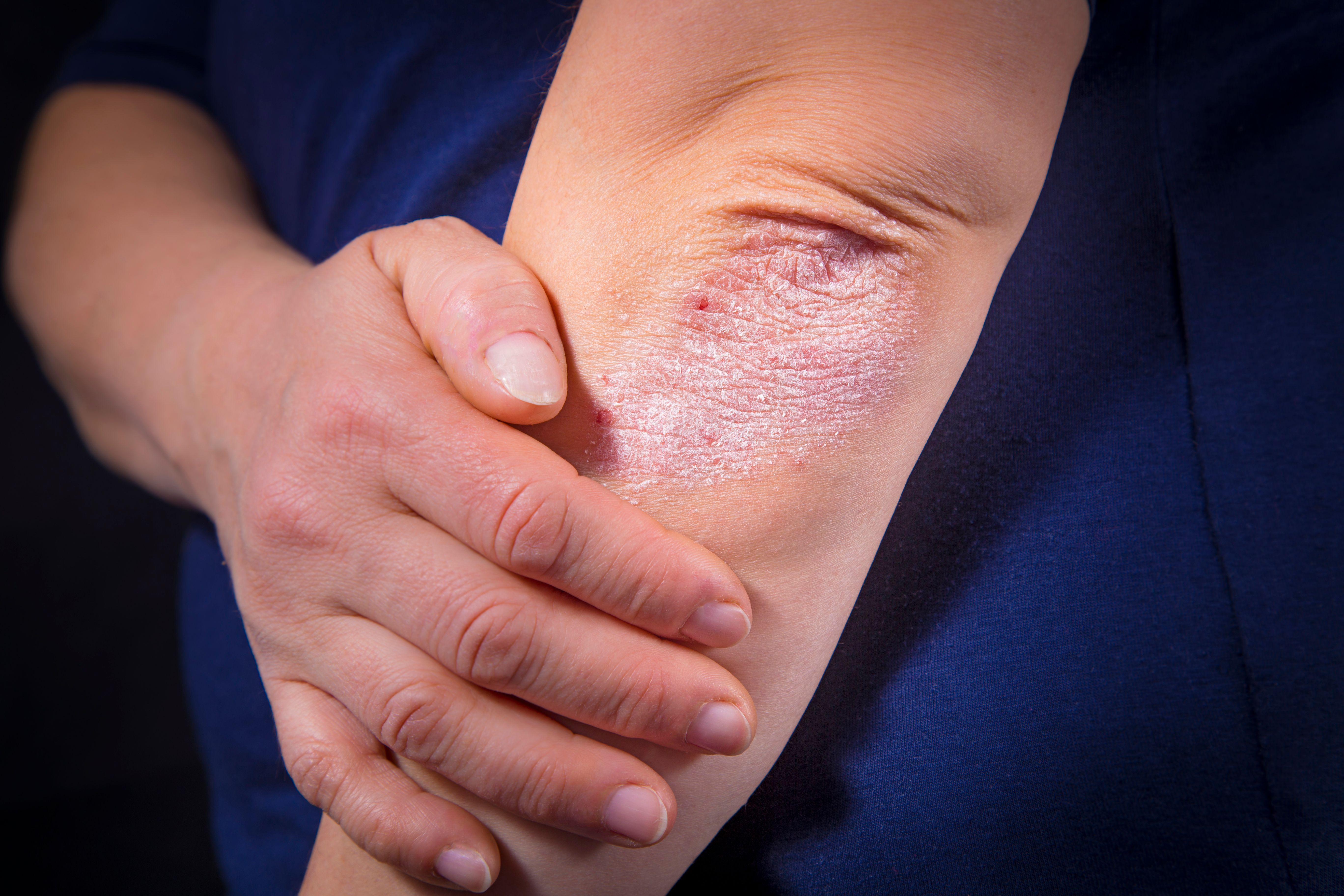
The tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu; Bristol Myers Squibb) was approved by the US Food and Drug Administration (FDA) in September 2022 for the treatment of patients with moderate to severe plaque psoriasis. Deucravacitinib offers a treatment option for patients who are candidates for systemic therapy of phototherapy with superior efficacy shown in phase 3 studies. TYK2 inhibitors inhibit TYK2, an intracellular signaling kinase that mediates signaling of interleukin (IL)-12, IL-23, and type I interferon.
Also recently approved by the FDA are 2 novel, nonsteroid, topical treatment options, an exciting development for plaque psoriasis patients who may require long-term topical therapy to manage their condition. The first is tapinarof (Vtama; Dermavant) 1% cream, an aryl hydrocarbon receptor agonist that is responsible for decreasing IL-17, one of the hallmark cytokines that drive psoriasis.
“This once-daily medication clears lesions effectively and quickly and demonstrated high tolerability and safety in patients.1 This topical is a really good, new option to have for our plaque psoriasis patients and is an exciting addition to our armamentarium,” said Jeffrey M. Cohen, MD, an assistant professor of dermatology and the director of the Psoriasis Treatment Program at the Yale School of Medicine in New Haven, Connecticut.
Roflumilast (Zoryve; Arcutis) is another new topical medication that was recently FDA approved for the treatment of plaque psoriasis in patients older than 12 years. According to Cohen, the topical PDE4 inhibitor is administered once a day and works well in clearing individual lesions. Patients report some local stinging following application; however, this adverse effect is manageable and patients overall tolerate the topical cream very well.
One of the great advantages of these 2 new topical medications is that they are nonsteroidal, which allows for a simplistic treatment regimen that is different from the complicated scheming regimens needed with corticosteroid therapies.
“Both tapinarof and roflumilast can be used once a day on any area of the body for as long as needed, and that’s something that really none of the topical steroids can offer,” Cohen said. “This has clear, long-term therapeutic implications in that these nonsteroidal creams can circumvent the adverse events associated with the long-term use of corticosteroids.”
Corticosteroids largely remain the workhorse for providers treating patients with plaque psoriasis. In addition, topical calcineurin inhibitors used off-label, including tacrolimus and pimecrolimus, as well as vitamin D analogue creams such as calcipotriene are all effective psoriatic treatments and are particularly useful when administered intermittently during a corticosteroid drug holiday.
Oral deucravacitinib and the topical roflumilast and tapinarof creams are welcome additions to the dermatologic armamentarium. Roflumilast and tapinarof represent alternatives to topical corticosteroids; however, Cohen said that using a strong steroid on a plaque psoriasis lesion for a couple of weeks can often be effective and if the lesion clears, either roflumilast or tapinarof cream could be implemented as a maintenance therapy and for occasional flares.
“We are also seeing data from trials with new medications such as bimekizumab, a novel receptor blocking medication that inhibits IL-17A and IL-17F. In the phase 3 trials,2 it appears to be extraordinarily effective in clearing psoriasis lesions. The novel drug has already been approved in Europe for the treatment of moderate to severe psoriasis, so we are very hopeful that the FDA will approve this medication here in the US in the near future, although the timeline is not clear,” said Cohen.
With all the newly available systemic and topical therapies for psoriasis and those in the pipeline, it can be challenging for providers to navigate through increasing number of treatment options. When considering potential biologic or systemic treatments, it is crucial to review the patient’s comorbidities, according to Cohen, as well as understand which medications are better for psoriatic arthritis, inflammatory bowel disease, or other concomitant existing medical conditions such as congestive heart failure. Although there have been significant breakthroughs recently in psoriasis therapy, Cohen said personalized treatment choices remain the holy grail in ideal patient care.
“There are so many great treatment options and selecting the right medication for each patient can sometimes be a challenge, and advances that improve our ability to do this will help both physicians and patients,” Cohen said.
During August Psoriasis Awareness Month and at any time when needed, the National Psoriasis Foundation (NPF) is a valuable resource for patients with psoriasis, according to Cohen. The NPF provides patient education as well as resources to help patients cope with their lifelong condition. The NPF has guidelines that providers can refer to when considering potential therapies, including ideal treatment and management strategies.
According to Cohen, there are a lot of data to suggest that psoriasis is undertreated in the US, and there are many patients who would benefit from new and effective medications but do not have access to them. This can be due to several factors, including the availability of dermatologists, insurance issues, safety concerns, affordability, or physicians not feeling comfortable managing patients with these medications.
“Patients who have been dealing with psoriasis for a very long time may have felt that their condition could not be treated based on the experiences they had many years ago. I like to encourage those people to reengage now because many of the new medications we have are very effective even for people with severe psoriasis that they’ve had for a very long time. We can help a lot of patients with these medications so that they can resume a happy and healthy life that we all deserve,” Cohen concluded.
Disclosures: None relevant
References
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385(24):2219-2229. doi:10.1056/NEJMoa2103629
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158(7):735-744. doi:10.1001/jamadermatol.2022.1185






