- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
A look at drug development from bench to pharmacy
Author(s):
The cost of clinical trials to get a dermatology drug to market may cost as much as $25 million, which is slightly above a median of $19 million, according to the latest research.
The cost of clinical trials to get a dermatology drug to market may cost as much as $25 million, which is slightly above a median of $19 million.
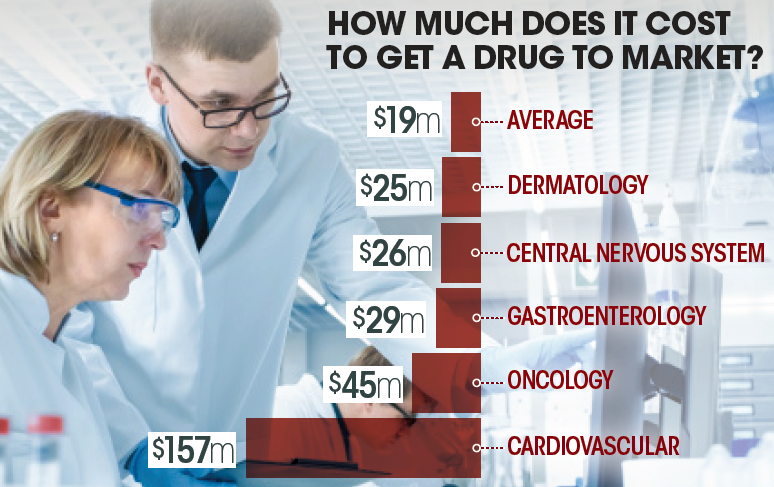
The cost of clinical trials to get a dermatology drug to market may cost as much as $25 million, which is slightly above a median of $19 million, researchers reported in JAMA Internal Medicine in September.
The study, by researchers at the Institute for Safe Medication Practices and Johns Hopkins University, analyzed the cost of drug trials for 59 drugs developed between 2015 and 2016.
While dermatology drugs costs on average $25 million, cardiovascular drugs averaged more than $157 million. Other fields associated with expensive trials included oncology, $45 million; gastroenterology, $29 million; and, neurology (central nervous system), $26 million. The more patients required for the trials, the more expensive the trial. “Pivotal clinical trial costs increased if more patients were needed to document treatment benefit, if active drug comparators were used, or to measure clinical end points rather than a change in a surrogate outcome,” researchers wrote. “The highest-cost trials were those in which the new agent had to be proved to be noninferior with clinical benefit end points compared with an agent already available or those that required larger patient populations to achieve statistical power to document smaller treatment effects or accrue infrequently occurring end points.”
(DOI:10.1001/jamainternmed.2018.3931)







