- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Fast-track designation for atopic dermatitis drug
Author(s):
Asana BioSciences announced that the U.S. Food and Drug Administration granted fast-track designation for the investigational ASN002, an oral treatment for moderate-to-severe atopic dermatitis. Learn more in this article.
The U.S. Food and Drug Administration granted fast-track designation for the investigational ASN002, an oral treatment for moderate-to-severe atopic dermatitis. (©TYLim/Shutterstock.com)
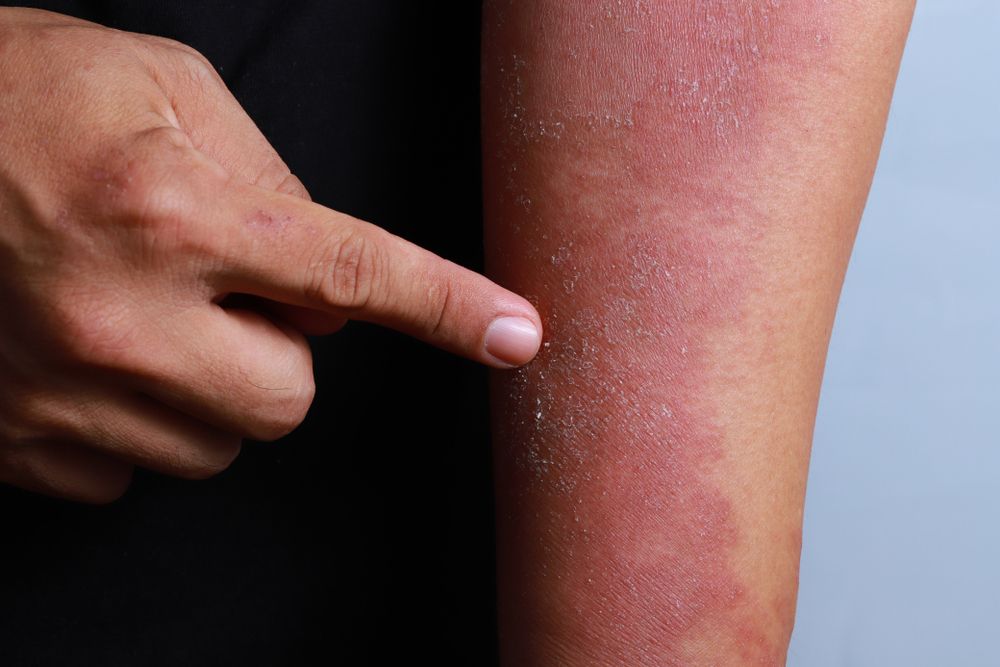
On Dec. 10, Asana BioSciences announced that the U.S. Food and Drug Administration granted fast-track designation for the investigational ASN002, an oral treatment for moderate-to-severe atopic dermatitis that is based on the inhibition of both a JAK and spleen tyrosine kinase (SYK) inhibitor.
“This designation recognizes the importance of accelerating the development of new medicines for the treatment of challenging dermatological/ inflammatory diseases that have a major impact on patients’ daily quality of life,” said Sandeep Gupta, founder and CEO of Asana.
ASN002 is currently under study in a phase 2b trial, RADIANT (Relief from Atopic DermatitIs with JAK and SYK INhibiTion-NCT03654755). And, in a phase 2 trial for patients with severe chronic hand eczema (NCT03728504). Asana reports that ASN002 is the first oral drug to demonstrate improvement in atopic dermatitis.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.






