- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
FDA approves novel topical retinoid trifarotene for acne
Author(s):
Today, the U.S. Food and Drug Administration approved the topical retinoid trifarotene 0.005% (AKLIEF, Galderma) for the treatment of acne vulgaris.
The once-daily topical retinoid targets retinoic acid receptor gamma (RARγ). (Frank - stock.adobe.com)
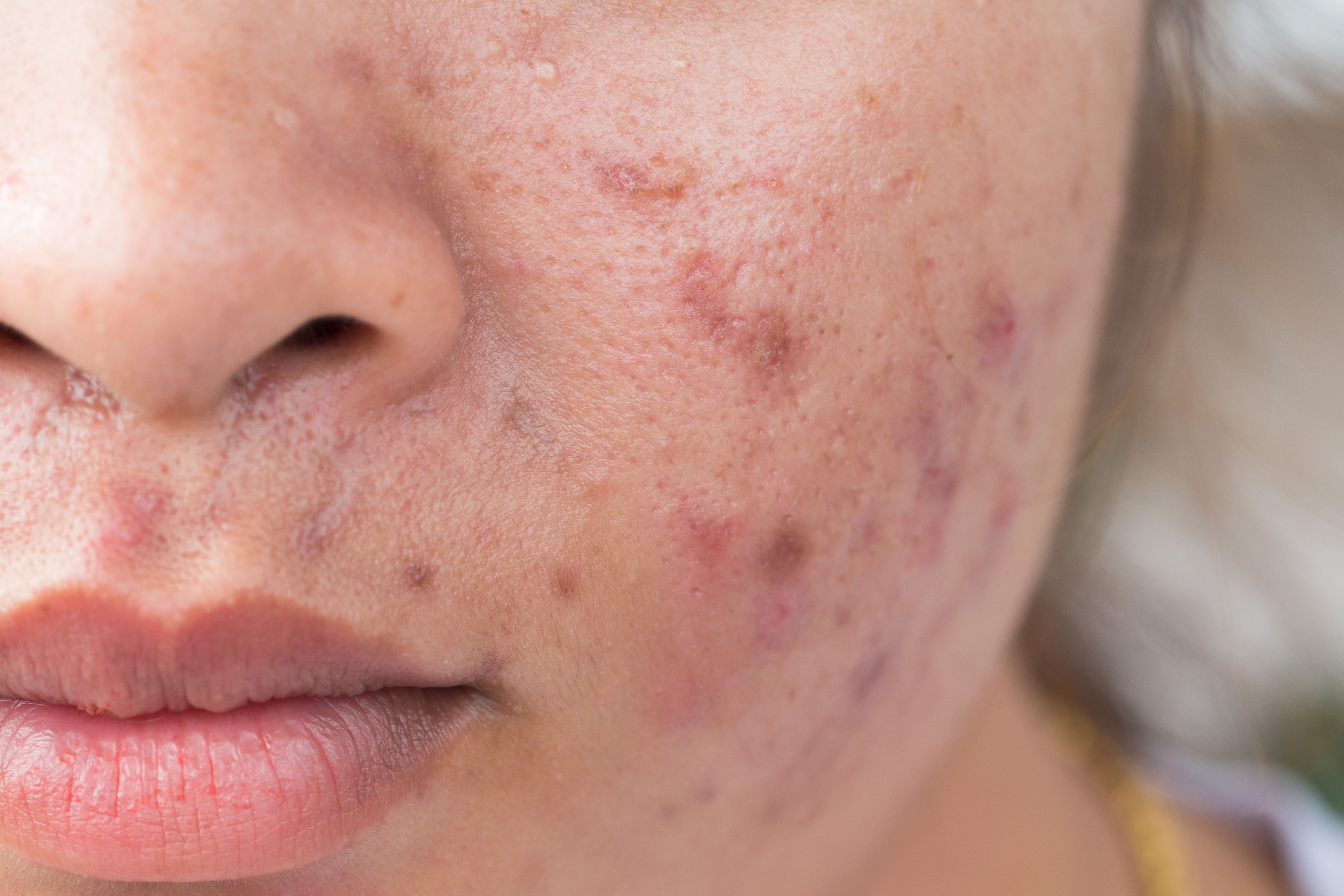
Today, the U.S. Food and Drug Administration (FDA) approved the topical retinoid trifarotene 0.005% (AKLIEF, Galderma) for the treatment of acne vulgaris.
“AKLIEF Cream is the first new retinoid molecule to be FDA approved for the treatment of acne in over 20 years and is the first topical treatment specifically studied to treat both facial and truncal (chest, shoulders and back) acne,” says Sandra Johnson, M.D., an investigator in the clinical trials of AKLIEF Cream and a dermatologist at Johnson Dermatology in Fort Smith, Ark. “Additionally, AKLIEF Cream is potent and effective at low doses, making it suitable for use across large areas. It is also the only topical retinoid that specifically targets retinoic acid receptor gamma (RAR-gamma), the most common retinoic acid receptor found in the skin."
READ MORE: What you may have missed about trifarotene
Trifarotene 0.005% is expected to be available in the United States in November 2019. It will be provided in a 45-gram pump, according to Galderma.
Researchers studied the topical’s safety and efficacy, publishing the results of two phase 3 studies PERFECT 1 and PERFECT 2 in the June 2019 Journal of the American Academy of Dermatology. The paper details results from two 12-week clinical trials of once daily trifarotene versus vehicle in patients 9 years and older with moderate facial and truncal acne. In total, more than 2,400 were studied at 200 sites globally.
Outcomes from trifarotene were significantly superior to vehicle in reducing inflammatory and noninflammatory lesion counts on the face and trunk, the authors report. This is one of the few studies in the literature to report outcomes of acne treatment to the chest in back, despite that truncal acne is thought to occur in about 56% of acne patients, according to the paper.
Local irritation related to trifarotene included erythema, scaling, dryness and stinging and burning - side effects that were mostly mild to moderate, transient and consistent with topical retinoid dermatitis. Some of the subjects discontinued trifarotene due to adverse events, including 1.9% in PERFECT 1 and 1.2% in PERFECT 2.
“There were no relevant changes in laboratory safety test results when trifarotene 0.005% was applied to large surface areas,” according to the authors of the JAAD study.
In a 52-week study published July 15, 2019 in the Journal of the European Academy of Dermatology and Venereology, researchers looked at the long-term safety and efficacy of trifarotene in facial and truncal acne. Researchers of the multicenter, open-label study of 342 patients with moderate facial and truncal acne found the topical retinoid to be well tolerated and effective.
READ MORE: View the company press release here
Trifarotene treatment resulted in adverse events in 12.6% of patients, though none were serious. Most treatment-related adverse events were cutaneous and occurred in the first three months.
At week 12, the Investigator Global Assessment success rate was 26.6% and the Physician’s Global Assessment 38.6%. Success rates increased to 65.1% for the Investigator Global Assessment and 66.9% for the Physician’s Global Assessment at Week 52, according to the study.
Trifarotene has been studied in acne patients 9 years of age and older. Safety and effectiveness have not been established in pediatric populations under the age of 9 years, according to Dr. Johnson.
“From my personal experience as an investigator I would recommend this treatment for individuals who feel self-conscious of the impact of facial and truncal acne and are seeking a solution,” Dr. Johnson says. “AKLIEF Cream can be used as a standalone treatment.”
Dermatologists should know that adverse reactions to trifarotene are most likely to occur during the first 4 weeks of treatment and may decrease with continued use, according to Dr. Johnson.
“In studies, patients were encouraged to use a non-comedogenic and hypoallergenic moisturizer of their choice but not to apply moisturizer one hour before or one hour after AKLIEF Cream application to help alleviate skin irritation. It’s important for patients to work with their healthcare provider to determine an approach that works best for them,” she says.
As a pipeline drug, trifarotene stood out as a promising topical retinoid that could help providers better manage acne and related sequelae, as author Anna L. Chien, M.D., noted in a review of retinoids in acne management published December 2018 in the Journal of Drugs in Dermatology.
“This medication is the first fourth generation topical retinoid with potent and selective RARγ agonist activity with minimal RARβ-mediated effects, thus lending it greater efficacy with potentially decreased side effect of skin irritation. Furthermore, trifarotene has also been shown to possess increased hepatic instability compared to first and third generation retinoids, thus theoretically giving it a more tolerable systemic safety profile,” Dr. Chien writes.
Disclosures:
Dr. Johnson has served as an investigator, consultant, and speaker for Galderma.






