- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Dermatology Times
Oral Therapies Offer New Options for Patients With Psoriasis
Author(s):
Ben Lockshin, MD, FAAD, shared his insights on the latest advancements in oral options for psoriasis therapies.
Image Credit: © Daniel Beckemeier
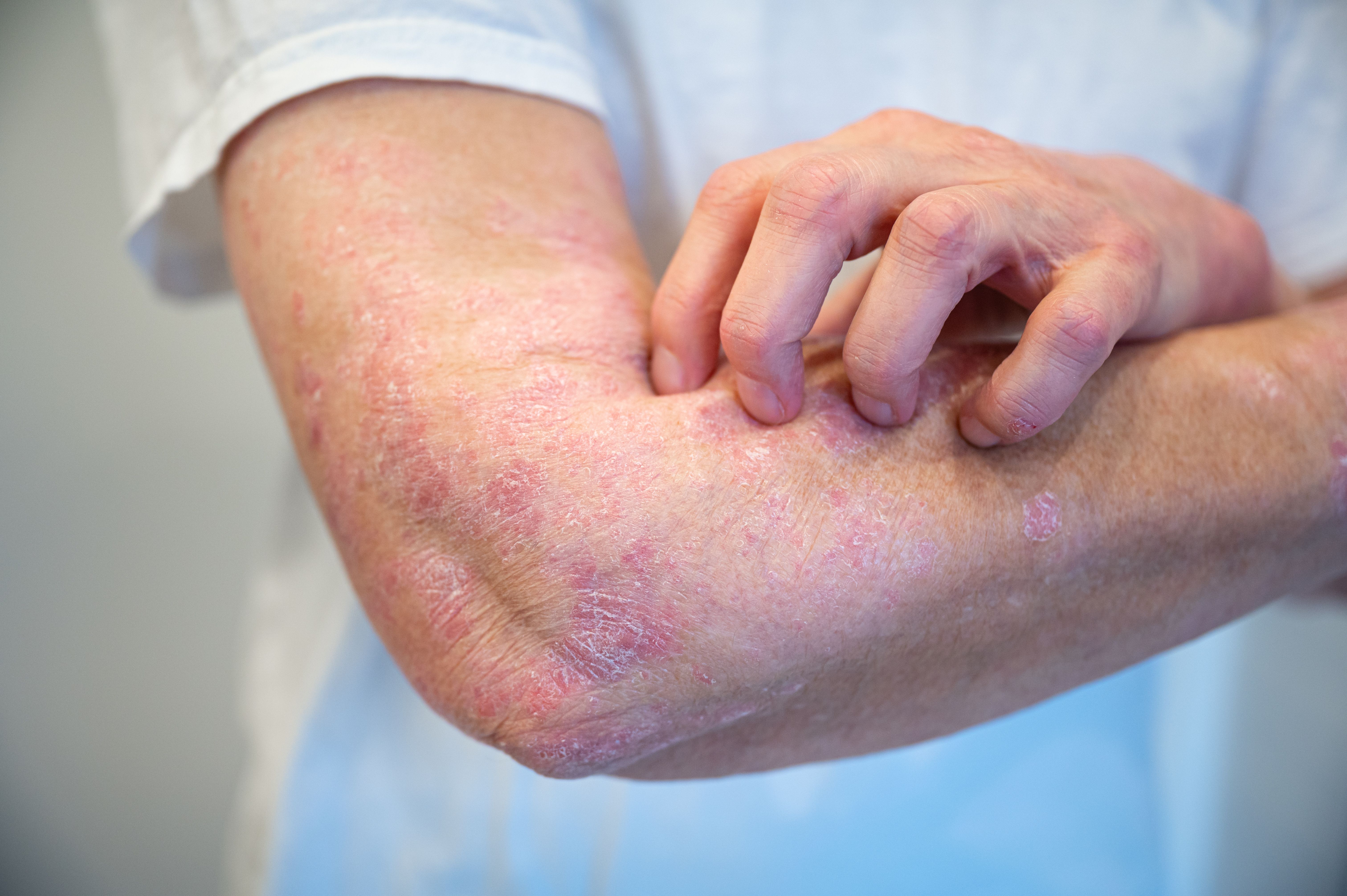
In the ever-evolving landscape of psoriasis treatment, oral therapies continue to play a crucial role. Ben Lockshin, MD, FAAD, assistant professor at Georgetown University’s department of dermatology and director of the Clinical Trials Center at US Dermatology Partners in Washington, DC, shared his insights on the latest advancements in oral options for psoriasis therapies during the session “Oral Options for Psoriasis Therapies” at the 2024 Fall Clinical Dermatology Conference for PAs and NPs in Scottsdale, Arizona.1 In an interview with Dermatology Times, Lockshin explained how far we have come in oral treatment options for psoriasis, the future outlook, and patient populations who benefit most from oral therapies.
Classic Oral Treatments
The classic oral treatments for psoriasis include methotrexate, cyclosporine, and fumaric acid. These medications have been staples in psoriasis management, particularly in countries such as Germany, where they are frequently prescribed for moderate to severe cases. Fumaric acid, for example, has demonstrated efficacy with a Psoriasis Area and Severity Index (PASI) 75 response rate of 40.3% and a PASI 90 response rate of 22.3%. PASI scores are critical in assessing the effectiveness of psoriasis treatments, with PASI 75 and PASI 90 indicating 75% and 90% improvement, respectively.2
Newer-Generation Oral Therapies
“We live in an exciting time because we’ve got new topical therapies, multiple oral therapies, and an array of biologic agents, and patient preferences change,” Lockshin said. The newer generation of oral therapies includes apremilast (Otezla; Amgen) and deucravacitinib (Sotyktu; Bristol Myers Squibb). Apremilast is an oral phosphodiesterase 4 inhibitor that modulates the inflammatory response. It is approved for psoriasis, psoriatic arthritis, and oral ulcers associated with Behçet disease.3 Deucravacitinib, a more recent addition, has shown promising results in terms of efficacy and tolerability.4
Clinical Data Supporting Apremilast
In findings from a phase 3 trial involving 595 patients with mild to moderate plaque psoriasis inadequately controlled or intolerant to at least 1 topical therapy, apremilast demonstrated significant improvements. At week 16, the primary end point of clear or almost clear skin, as measured by a point reduction of 2 or more in static Physician Global Assessment scores, was achieved.5
Pediatric Use of Apremilast
Apremilast has also been evaluated in pediatric patients aged 6 to 17 years with moderate to severe plaque psoriasis. Findings from a study involving 245 patients showed significant improvements at 16 weeks, confirming its efficacy and safety in younger populations.6
Apremilast vs Deucravacitinib
Deucravacitinib offers the convenience of once-daily dosing and has demonstrated superior efficacy to apremilast, with a similar adverse event profile. Findings from phase 3 trials (POETYK-PSO-1 [NCT03624127] and POETYK-PSO-2 [NCT03611751]) showed that deucravacitinib sustained PASI 75 and PASI 90 responses well from week 52 through 4 years, making it a compelling option for long-term management, according to investigators.7
Both apremilast and deucravacitinib have manageable safety profiles. Long-term data for deucravacitinib indicate that rates of major adverse cardiovascular events and other serious conditions are comparable to those seen with other psoriasis treatments. Regular monitoring is essential, but these therapies are generally safe for most patients.7
Oral Options vs Injectables
The choice between oral and injectable treatments often depends on patient preference and specific clinical scenarios. Some patients prefer the convenience of oral medications, whereas others may opt for injectables due to their efficacy. Comparative studies, such as the IMMpulse phase 4 study, are providing valuable data on patient outcomes and preferences.8
“I like oral therapies to initiate prior to thinking about a biologic,” Lockshin explained. “There are 3 patient types that are great for oral therapies. One is the dabblers: the patients who are tepid about starting systemic therapy. Given the fact that it’s a small molecule, there’s less of a chance of immunogenicity. There’s a possibility [that] if they want to stop, they can stop and then restart without much concern. In addition, I like using orals for younger patient populations who don’t want to have injectable medications in their dormitory fridge or if they have roommates. So thinking about all options fits into their lifestyle a lot better. And the last one is for those patients with more mild disease. Oral options fit quite nicely into the treatment algorithms.”
Future of Oral Therapies
Several new oral therapies are in clinical development, aiming to enhance efficacy, safety, and patient quality of life. Lockshin concluded, “In this newer generation of managing patients with psoriasis, the introduction of new deucravacitinib allows us to achieve the same meaningful response rates as we do with some of the injectable medications with a once-daily pill. This has changed the way that we look at oral therapies and the management of patients with moderate [to] severe psoriasis.”
References
1. Lockshin B. Oral options for psoriasis therapies. Presented at: 2024 Fall Clinical Dermatology Conference for PAs and NPs; May 31-June 2, 2024; Scottsdale, AZ.
2. Atwan A, Ingram JR, Abbott R, et al. Oral fumaric acid esters for psoriasis. Cochrane Database Syst Rev. 2015;2015(8):CD010497. doi:10.1002/14651858.CD010497.pub2
3. Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37-49. doi:10.1016/j.jaad.2015.03.049
4. Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40-51. doi:10.1016/j.jaad.2022.08.061
5. Stein Gold L, Papp K, Pariser D, et al. Efficacy and safety of apremilast in patients with mild-to-moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2022;86(1):77-85. doi:10.1016/j.jaad.2021.07.040
6. Fiorillo L, Becker E, de Lucas R, et al. Efficacy and safety of apremilast in pediatric patients with moderate-to-severe plaque psoriasis: sixteen-week results from SPROUT, a randomized controlled trial. J Am Acad Dermatol. 2024;90(6):1232-1239. doi:10.1016/j.jaad.2023.11.068
7. Strober B, Blauvelt A, Warren RB, et al. Deucravacitinib in moderate-to-severe plaque psoriasis: pooled safety and tolerability over 52 weeks from two phase 3 trials (POETYK PSO-1 and PSO-2). J Eur Acad Dermatol Venereol. Published online March 7, 2024. doi:10.1111/jdv.19925
8. Stein Gold LF, Bagel J, Tyring SK, et al. Comparison of risankizumab and apremilast for the treatment of adults with moderate plaque psoriasis eligible for systemic therapy: results from a randomized, open-label, assessor-blinded phase IV study (IMMpulse). Br J Dermatol. 2023;189(5):540-552. doi:10.1093/bjd/ljad252






