- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Melanoma subtypes challenging to diagnose, treat
Author(s):
Lentigo maligna (LM) and acral lentiginous melanoma (ALM) can be challenging to diagnosis and treat, according to experts. Here, they offer insight into incidence, presentation, and management options. Learn more
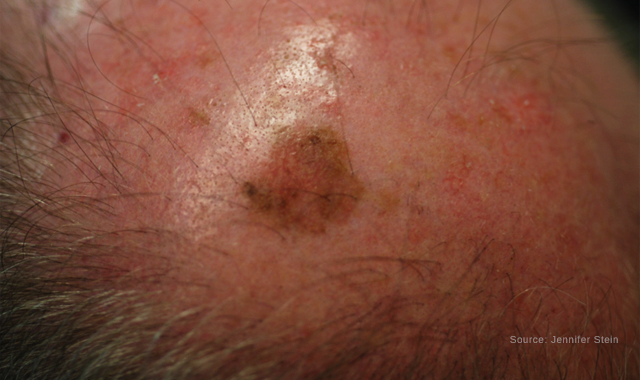
Lentigo Maligna of the scalp
Lentigo maligna (LM) and acral lentiginous melanoma (ALM) can be challenging to diagnosis and treat, according to experts. Here, they offer insight into incidence, presentation, and management options.
READ: Survival with melanoma subtypes varies
“Diagnosing early melanomas in these anatomical locations can be challenging,” according to Clara Curiel-Lewandrowski, M.D., associate professor of medicine and dermatology, and clinical director, Cutaneous Oncology Program, Skin Cancer Institute, University of Arizona, Tucson, Ariz. “It is important to examine the hands and feet, including the nails, and to closely evaluate and monitor pigmented lesions that are new or appear to be changing in these anatomic locations.”
Presentation patterns
LM usually presents in chronically sun-exposed areas, including the face (i.e., nose, cheek and forehead), neck and dorsal hands. It appears as a slow-growing light brown to black lesion with irregular borders. The pigmentation can be similar throughout the lesion or with areas of color variegation, explains Dr. Curiel-Lewandrowski, M.D.
READ: Viruses may lead to skin disease, cancer
Acral lentiginous melanoma (ALM), in comparison, presents on palms, soles (more common than hands) or beneath the nail (subungual melanoma).
“It can arise de novo in normal-appearing skin, or it can develop from a pre-existing melanocytic nevus,” she says.
ALM usually starts as a slowly-enlarging flat macule of pigmented skin. “The presence of melanoma cells within the epidermis can persist in this location for months or years before it becomes invasive,” Dr. Curiel-Lewandrowski says. “Nodular melanomas can also evolve in these anatomical locations demonstrating a more rapid progression.”
Oftentimes, patients initially believe ALM is simply an insignificant area of depigmentation. The color varies from a mixture of brown and blue-grey or black and red. It can also be pinkish/red, or have no pigment.
Typically, ALM measures several centimeters in diameter. At first, ALM has a smooth surface because the epidermal layer is relatively thicker in these locations. It later becomes thicker with an irregular surface that may become warty; ulceration or bleeding may occur.
NEXT: Population prevalence
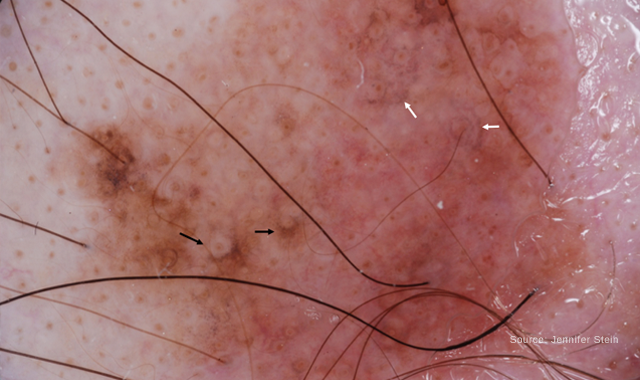
Dermoscopy of Lentigo Maligna. The black arrows highlight areas of asymmetric pigmentation around follicular openings. The white arrows show grey dots around follicular openings.
Population prevalence
Individuals with a light skin complexion (i.e., skin phototype 1 and 2), account for 93% of LM cases. A history of chronic skin damage is the disease’s primary driving cause. Men are more commonly diagnosed than women (men, 55%; women, 44%), with 61 years as the mean age of diagnosis.
READ: Melanoma rates rise in Hispanics
ALM, in comparison, is equally common in males and females and the majority of cases arise in people over age 40.
While ALM has a similar incidence between different skin types, it disproportionately affects skin of color.
“Patients with darker skin types have fewer superficial spreading melanomas than patients with lighter skin,” says Jennifer Stein, M.D., Ph.D., associate professor, New York University School of Medicine, New York, N.Y., says. “As a result, ALM accounts for a larger percentage of their melanomas.” In the United States, the age-adjusted incidence rate of ALM is 1.8 per million person-years.
Dr. Curiel-Lewandrowski points out that melanoma in situ (MIS) LM type, a subtype of MIS, is increasing worldwide. “It is unclear if this is due to an increase in incidence, improved early detection, changes in histological criteria or increased reporting to cancer registries,” she says.
In the United States, the incidence of MIS LM type has increased 9.5% annually, representing one of the fastest growing malignancies in The Surveillance, Epidemiology, and End Results (SEER) Program database.
Dr. Curiel-Lewandrowski points to an ongoing controversy on the use of LM and MIS LM type terminology. In the case of MIS LM type confluent nests of atypical melanocytes at the epidermal level are usually described. Most organizations and reporting sites will not make a distinction between LM and MIS LM type, with both entities being described and reported under MIS.
Cancer causes
Cumulative solar ultra-violet light exposure is the key cause of LM. While there is no hallmark or predominant genetic signature for LM, some reports have suggested an increased prevalence of MC1R and KIT mutations, with BRAF V600K being more predominant than BRAF V600E mutations in this subgroup, Dr. Curiel-Lewandrowski reports.
READ: Skin clues help identify cancer metastases
In comparison, it is not clear what causes ALM. Notably, there is an increased prevalence of c-KIT mutation in this subtype. But AML in situ is not dangerous; it only becomes potentially life threatening if an invasive melanoma develops within it.
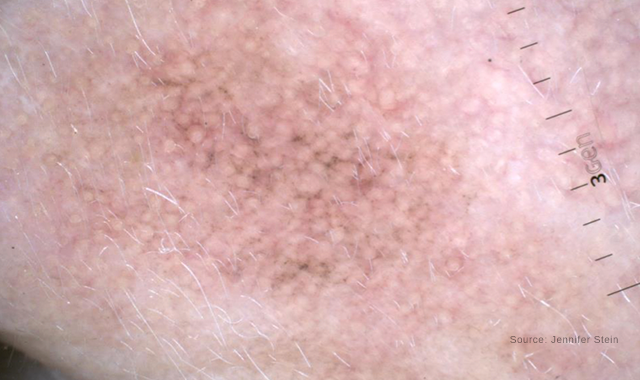
Dermoscopic image of lentigo maligna demonstrating grey pigment around follicular openings.
Diagnostic challenges
Patients presenting with an atypical or changing pigmented lesion on sun-exposed areas require clinical evaluation. Tools for non-invasive assessment of the lesion include clinical inspection, dermoscopy, in vivo confocal microscopy and other non-invasive technologies such as Melafind, epidermal genetic information retrieval technology (DermTech), and Nevisense (which is currently under U.S. Food and Drug Administration [FDA] approval review).
READ: Technology advances melanoma diagnosis, treatment
“The decision on whether to biopsy primarily relies on unaided clinical exam and dermoscopy evaluation,” Dr. Curiel-Lewandrowski says. Dermoscopic clues for LM are pigmentation around hair follicles and the colors grey and pink.
Because a significant proportion of LM lesions present in cosmetically sensitive areas and 77% of them exceed 6 mm in size, an excisional biopsy can be a challenge because of the tradeoff for large surgical defects and repairs in these anatomical locations.
“Implementation of multiple incisional biopsies, guided by dermoscopy, is a reasonable approach to minimize sampling bias and to maximize the likelihood of accurate diagnosis while decreasing the comorbidity of an excisional surgical procedure,” Dr. Curiel-Lewandrowski says. “Be aware of sampling bias when performing incisional biopsies of larger pigmented lesions.”
Dr. Stein says dermoscopy can also be useful to distinguish ALM from acral nevi. “The parallel ridge pattern is highly specific for ALM, so be sure to biopsy any lesion with this pattern,” she says. “Typical parallel furrow, fibrillar or lattice patterns are very reassuring. Once a lesion is larger than 7 mm, and especially once it’s larger than 1 cm, have a higher index of suspicion and watch out for an irregular blotch or other asymmetry in an otherwise typical pattern.”
When subungula melanomas start in the matrix, they may present as diffuse discoloration or irregular pigmented longitudinal band(s) on the nail plate, Dr. Curiel-Lewandrowski says. Advanced melanoma destroys the nail plate altogether and in some instances can be amelanotic.
READ: Intralesional therapy for metastatic melanoma
As a word of caution, when performing a biopsy make sure to use a thorough sample to minimize the risk of sample bias and misdiagnosis, she adds.
NEXT: Recommended treatments, challenges of LM
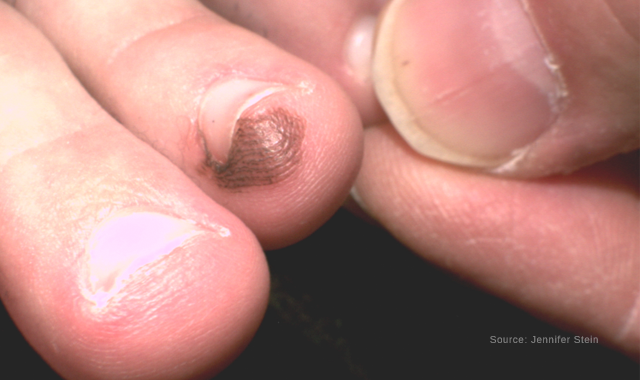
Acral congenital nevus
Recommended treatments, challenges of LM
Surgery continues to be the treatment of choice for LM. A surgical excision with a 5 mm margin is the current recommendation for an MIS LM type diagnosis, which is based on level C evidence.
READ: Targeting your melanoma therapy
By performing surgical excisions, you can perform a proper histological evaluation and assess the margins in permanent sections. “This is important in the case of MIS LM type because between 5% and 30% of cases are upstaged to invasive melanoma in some series,” Dr. Curiel-Lewandrowski says.
Some of the challenges of surgical excision include larger lesions and often encountering positive margins after excision. To avoid the latter, Dr. Curiel-Lewandrowski suggests performing one or a combination of the following approaches: small scouting punch biopsies to evaluate the surrounding normal appearing skin for the presence of atypical melanocytes suggestive of subclinical disease, staging surgical excisions, using wood lamp examination, using the spaghetti technique and when available and feasible margin evaluation by in vivo confocal microscopy.
While Mohs surgery has been used with some success, clearance rates due to the difficulty of accurately identifying melanocytes on frozen sections have been reported. However, reasonable outcomes have been reported in the hand of experienced skilled Mohs surgeons when immunohistochemistry is applied to aid in the identification of atypical melanocytes in the frozen tissue specimen.
Although it is not approved by the FDA for the treatment of melanoma, multiple studies support the use of imiquimod cream for the treatment of LM in patients who cannot tolerate surgery, Dr. Stein reports. In fact, a recent study1 showed that applying imiquimod at least five times a week and more than 60 times led to better clearance.
Imiquimod is an immune response modifier that works by activating Toll-like receptor 7 and enhancing immune response to the regional site where the drug is applied, Dr. Curiel-Lewandrowski explains. Response rates to imiquimod are variable.
Consider a combination of imiquimod with tazarotene for patients who aren’t surgical candidates due to advanced age or medical comorbidities, patients declining surgical intervention, or in the case of large lesions with the objective of reducing the need for large surgical defects (neoadjuvant), Dr. Curiel-Lewandrowski advises.
READ: Reprogramming cancer with a code
A study2 of 46 MIS LM type patients suggests that those who used imiquimod 5% five times a week and tazarotene 0.1% twice weekly for three months preceding staged excision resulted in lower evidence of residual disease compared to those who were treated with imiquimod alone (22% of lesions versus 36%).
Other alternatives to imiquimod in challenging cases include intralesional interferon alpha and radiation treatment. However, limited evidence exists to support these alternatives.
Laser treatment, another option for MIS LM type, has high recurrence rates. “Use caution when performing laser treatment in lentigines that display some degree of atypical features and consider reassessing lesions that recur in a short time,” Dr. Curiel-Lewandrowski says.
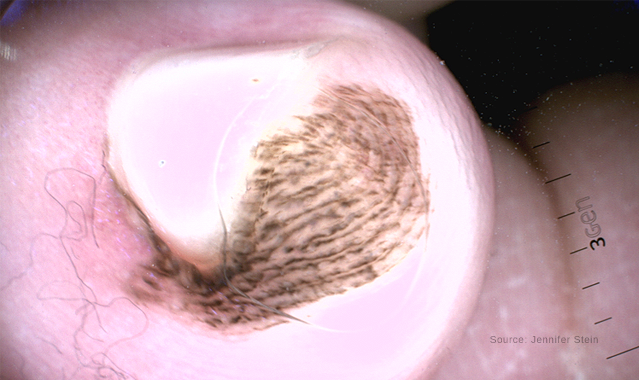
Dermoscopy of an acral congenital nevus demonstrating a benign pattern
Recommended treatments, challenges of ALM
Like LM, surgical excision is the initial treatment protocol for ALM. “The lesion should be completely excised with a reasonable surgical margin considering the anatomical structures,” Dr. Curiel-Lewandrowski says. “Further treatment depends mainly on the lesion’s thickness and indication for sentinel lymph node biopsy.”
In some occasions, a flap or graft to close the wound is necessary. “In the case of ALM and subungual melanoma, this may include partially amputating a digit,” Dr. Curiel-Lewandrowski continues. “Occasionally, the histological assessment will report incomplete excision of the melanoma, despite wide margins. This means that further surgery or radiotherapy will be recommended to ensure that the tumor is completely removed.”
READ: Personalized Melanoma Vaccines Debut in Humans
In general, ALM treatment follows a similar indication as other subtypes of melanoma depending on the disease’s stage. Somatic mutations within the c-KIT gene have been detected in 10.8% of Chinese melanoma patients.3
“Because the prevalence of c-KIT mutation has been reported in this melanoma subtype, treatment with c-KIT inhibitors represent a therapeutic option for eligible patients with advanced disease,” Dr. Curiel-Lewandrowski says.
Imatinib, a selective inhibitor targeting Abl as well as c-KIT and the platelet-derived growth factor receptor, has been tested for the efficacy and toxicities in metastatic melanoma patients, suggesting that imatinib may increase the progression-free survival and overall survival in selected melanoma patients harboring mutations in the c-KIT gene,4 Dr. Curiel-Lewandrowski reports.
Dr. Stein also notes that ALM can have extensive subclinical extension and skip areas, making it difficult to clear the margin.
Knowing what to look for when diagnosing LM and ALM is key when diagnosing these special site melanomas. A variety of treatments are available, with surgery being the most common. Know the pros and cons of other options.
Disclosures: Dr. Stein reports no relevant disclosures. Dr. Curiel-Lewandrowski is a DermSpectra Inc, stockholder. She reports no conflict of interest with the material discussed in this article.
References
1. Mora AN, Karia PS, Nguyen BM. A quantitative systematic review of the efficacy of imiquimod monotherapy for lentigo maligna and an analysis of factors that affect tumor clearance. J Am Acad Dermatol. 2015 Aug;73(2):205-212. doi: 10.1016/j.jaad.2015.05.022. Epub 2015 Jun 16.
2. Hyde MA, Hadley ML, Tristani-Firouzi P, Goldgar D, Bowen GM. A randomized trial of the off-label use of imiquimod, 5%, cream with vs without tazarotene, 0.1%, gel for the treatment of lentigo maligna, followed by conservative staged excisions. Arch Dermatol. 2012 May;148(5):592-6. doi: 10.1001/archdermatol.2012.270.
3. Si L, Guo J. C-kit-mutated melanomas: the Chinese experience.
Curr Opin Oncol. 2013 Mar;25(2):160-165.
4. Postow MA, Carvajal RD. Therapeutic implications of KIT in melanoma.
Cancer J. 2012 Mar-Apr;18(2):137-141. doi: 10.1097/PPO.0b013e31824b2404.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







