- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
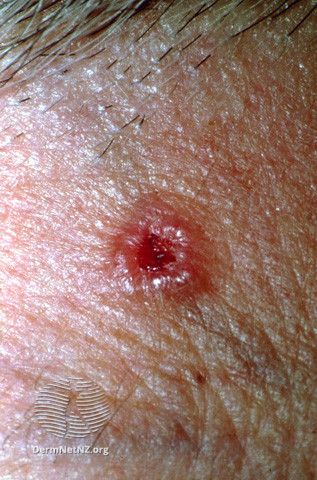
Medicus Pharma Submits Phase 2 IND Clinical Protocol to FDA to Non-Invasively Treat BCC
Author(s):
SKNJCT-003 is expected to enroll up to 60 patients.
Medicus Pharma recently announced its submission to the FDA of a phase 2 investigational new drug (IND) clinical protocol, SKNJCT-003, for the non-invasive treatment of basal cell carcinoma (BCC) using micro-array needles containing doxorubicin (D-MNA), developed by its wholly-owned portfolio company, SkinJect.
SKNJCT-003 is designed to be a randomized, double-blinded, placebo-controlled (P-MNA), multi-center study enrolling up to 60 patients presenting with nodular type BCC of the skin. The study will evaluate the efficacy of 2 dose levels of D-MNA compared to placebo in patients with nodular BCC. Patients will be randomized 1:1:1 to one of 3 groups: a placebo-controlled group receiving P-MNA, a low-dose group receiving 100μg of D-MNA, and a high-dose group receiving 200μg of D-MNA.
According to the announcement, the high dose of 200μg D-MNA is the maximum tolerated dose that was established in SkinJect's phase 1 safety and tolerability trial completed in March 2021. The phase 1 safety study, representing complete dissolution in 6 patients with BCC, demonstrated complete clinical response with no residual BCC and no relapse or recurrence across different dose levels and subject demographics.
"Our phase 2 study is designed to position the company to gain regulatory alignment and fast track the clinical development program," said Raza Bokhari, MD, the executive chairman and CEO of Medicus Pharma, in the news release. "BCC is perhaps the most common and fast-growing cancer across the globe with over 5 million cases diagnosed in the US each year. There is no effective non-invasive treatment regimen available to treat BCC; we aspire to deliver first-in-class, novel therapeutic alternative to treat this unmet medical need."
Reference
Medicus Pharma Ltd. submits to the FDA phase 2 IND clinical protocol to non-invasively treat basal cell carcinoma of the skin. News release. Medicus Pharma. January 3, 2024. Accessed January 3, 2024. https://medicuspharma.com/medicus-pharma-ltd-submits-to-the-fda-phase-2-ind-clinical-protocol-to-non-invasively-treat-basal-cell-carcinoma-of-the-skin/