- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
IL-17 inhibitors may fill treatment gap in rosacea
Author(s):
New options may be possible for treatment-resistant and severe rosacea with the use of IL-17 inhibitors as researchers conduct studies on effectiveness.
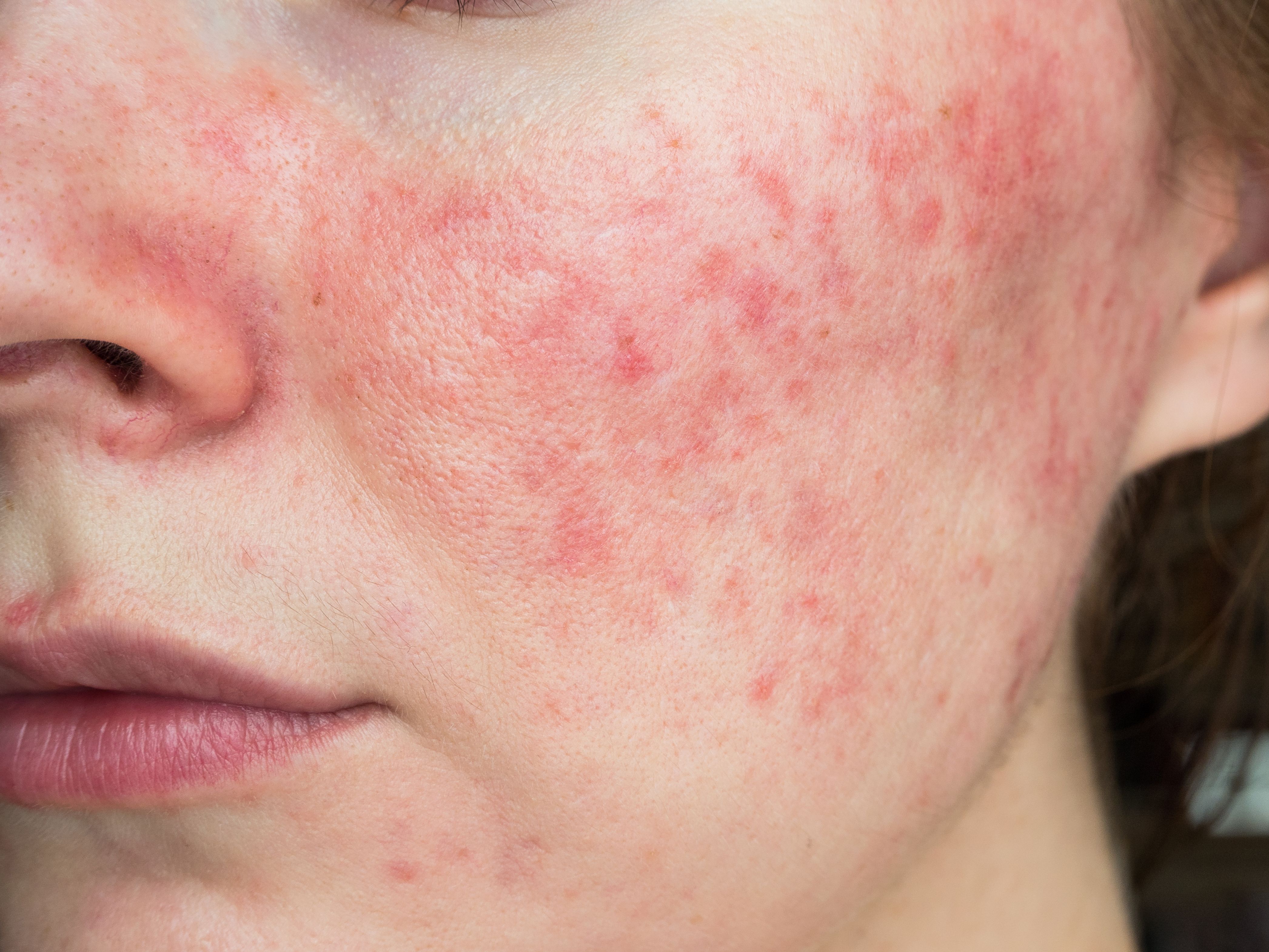
There’s need for therapies to address treatment-resistant and severe of papulopustular rosacea, and interleukin (IL) 17 inhibitors might just fit the bill, Canadian researchers report in a new review published August 12, 2019 in the Journal of Cutaneous Medicine and Surgery.1
Dermatologists primarily use standard rosacea treatments, including metronidazole, ivermectin, azelaic acid and low-dose oral antibiotics, to control symptoms with the medications’ anti-inflammatory effects. But these therapies also have this in common: they act at various stages along the IL-17 pathway, inhibiting IL-17’s downstream products or cytokines responsible for T helper (Th) 17 cell differentiation, according to the authors.
“Until recently, abnormal functioning of the innate immune system and neurovascular dysregulation have been at the forefront of the proposed [rosacea] pathophysiologies,” they write. “However, the role of adaptive immunity, IL-17 in particular, is slowly emerging.”
Among the findings that support IL-17’s role in rosacea, research by Buhl et al found Th1 and Th17 dominance in erythematotelangiectatic, papulopustular and phymatous rosacea, with highest T-cell activity and IL-17 immunostaining in papulopustular rosacea.2 The same authors reported on increased IL-6, tumor necrosis factor (TNF), IL-20 and CCL 29 gene expression - all of which are involved in IL-17 and IL-22 induction.
RELATED: New rosacea therapies show promise
Researchers also have reported that the skin of rosacea patients tends to have an increased density of Demodex folliculorum (D. folliculorum), which can lead to the release of more IL-17, according to the review in the Journal of Cutaneous Medicine and Surgery.
The authors point to gaps in treatment efficacy, including that there is no cure for rosacea and current treatment options lose effectiveness with increasing disease severity. Today’s topical and oral treatments relieve symptoms and slow disease progression, but they are associated with relapse when discontinued and treatments are lacking for severe and treatment-resistant papulopustular rosacea, they write.
Since IL-17 plays a pivotal role in papulopustular rosacea development and there are therapies on the market that target the IL-17 pathway, including secukinumab (Cosentyx, Novartis), ixekizumab (Talz, Lilly) and brodalumab (Siliq, Bausch Medical), IL-17 inhibitor drugs should be considered for the treatment of severe and treatment-resistant papulopustular rosacea, the authors reason.
First approved in January 2015, secukinuman has been available the longest, according to the paper. But Stanford University researchers are studying secukinumab, an antibody that binds specifically to IL-17A and has been shown to be effective in treating moderate-to-severe psoriasis, in a proof of concept study for moderate to severe papulopustular rosacea. The study was completed earlier this year, but results have not yet been posted, according to ClinicalTrials.gov.3
RELATED: Link between rosacea and systemic inflammatory diseases
Secukinumab might also be the safest of the three IL-17 inhibitors, according to the paper in the Journal of Cutaneous Medicine and Surgery. Brodalumab, a human monoclonal IgG2 antibody that binds to IL-17RA, has a black box warning for suicidal ideation and behavior; however, it might also show promise in the treatment of severe rosacea because it blocks the action of several IL-17 cytokines, according to the paper.
The high costs of these medications could prevent their use in diseases other than psoriasis and psoriatic arthritis - that’s even if further research reveals they are effective and safe for treatment of severe and treatment-resistant papulopustular rosacea, the authors write.
ÂDisclosures
The paper’s senior author Ronald Vender has received grants and/or research support from Abbvie, Amgen, Centocor, Dermira, Dermavant, Galderma, GSK, Leo, Lilly, Takeda, Novartis, Merck, Pfizer, Regeneron, UCB and participated in speakers bureaus and received honoraria from Abbvie, Amgen, Janssen, Galderma, GSK, Leo, Lilly, Novartis, Pfizer, Bausch-Health, Actelion, Celgene, Cipher, UCB. He has also received consulting fees from Abbvie, Amgen, Janssen, Galderma, GSK, Leo, Lilly, Novartis, Pfizer, BauschHealth, Actelion, Celgene, Cipher, UCB.
References
1. Amir ali A, Vender R, Vender R. The Role of IL-17 in Papulopustular Rosacea and Future Directions. J Cutan Med Surg. 2019;:1203475419867611.
2. Buhl T, Sulk M, Nowak P, et al. Molecular and Morphological Characterization of Inflammatory Infiltrate in Rosacea Reveals Activation of Th1/Th17 Pathways. J Invest Dermatol. 2015;135(9):2198-2208.
3. ClinicalTrials.gov[Internet].Bethesda(MD):NationalLibraryofMedicine(US).2000Feb29- .Identifier NCT03079531, Open label study to assess the effect of secukinumab in moderate to severe papulopustularrosacea;2017Mar14.Availablefrom:https://clinicaltrials.gov/ct2/show/NCT03079531