- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
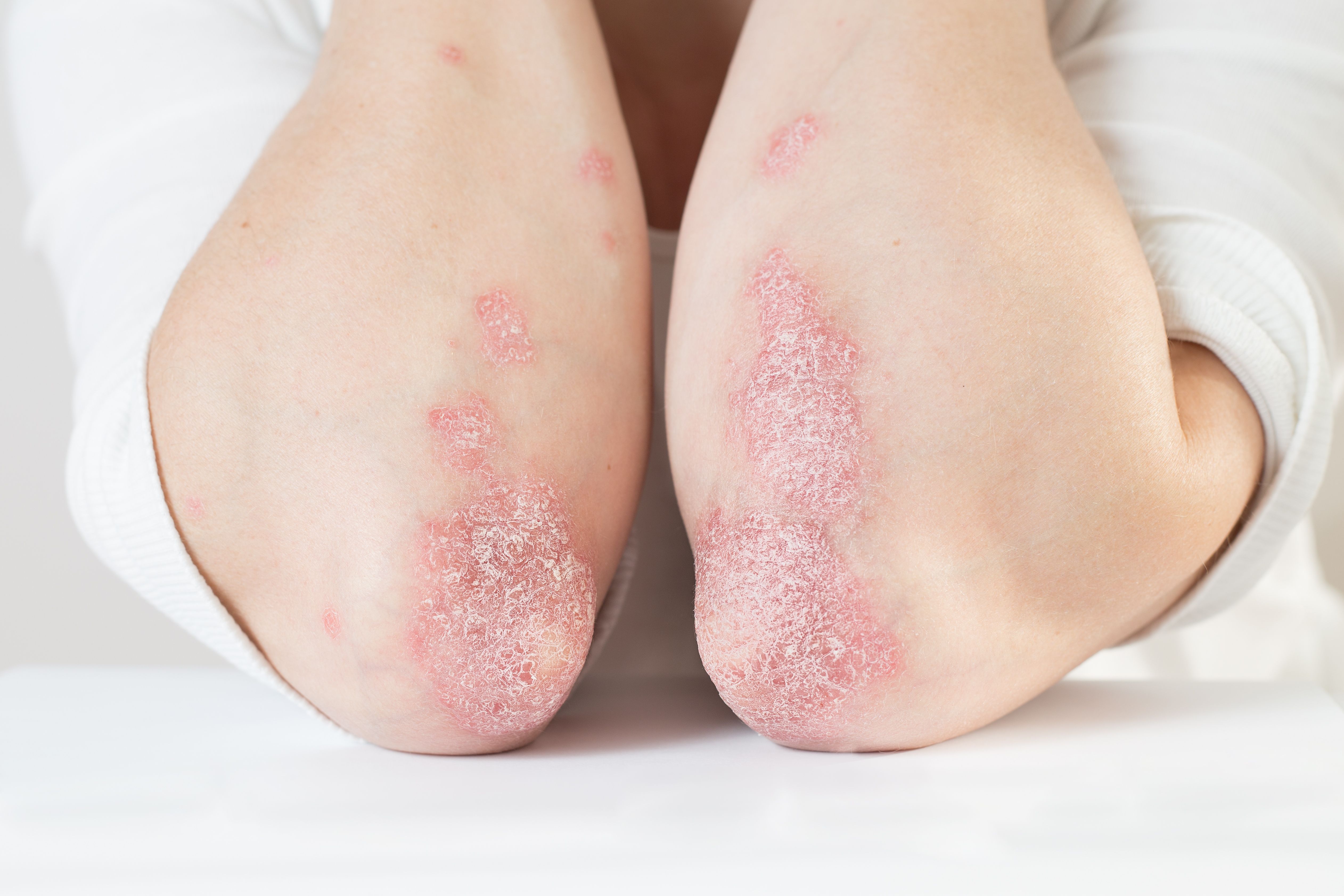
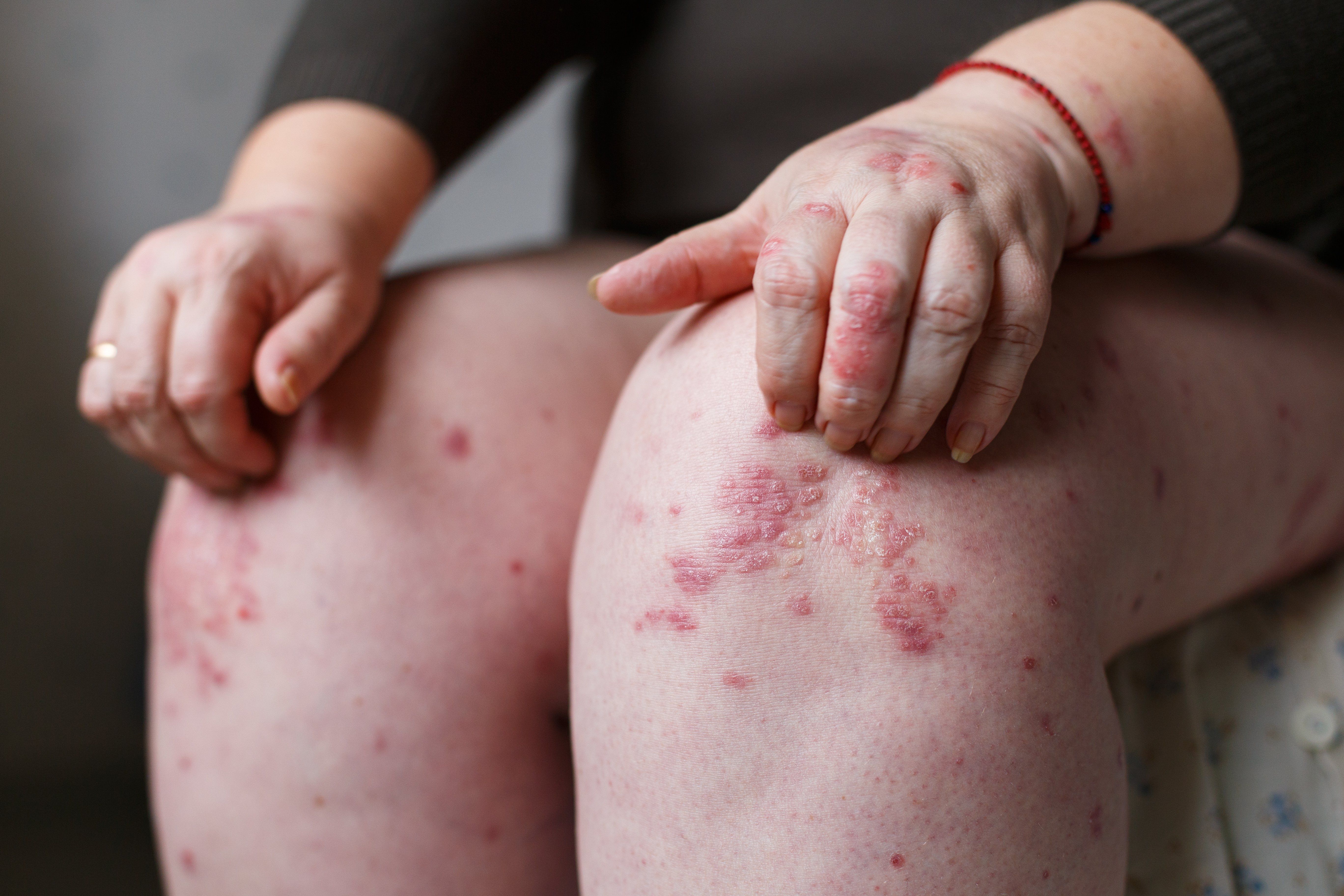
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Biologic guidelines for psoriasis let providers choose
Author(s):
When it comes to biologic drugs, the latest joint American Academy of Dermatology-National Psoriasis Foundation guidelines are less prescriptive than descriptive, offering a comprehensive discussion on the facts of each biologic to inform physicians’ selections.
Dr. Strober

When it comes to biologic drugs, the latest joint American Academy of Dermatology-National Psoriasis Foundation guidelines are less prescriptive than descriptive, comprehensively discussing the facts of each biologic to inform physicians’ selections.
RELATED: Meta-analysis points to most effective biologics for psoriasis
“The guidelines are not about choosing which drug is better than the next. They are more about laying out the data and use of these medications very thoroughly and allowing physicians to come to their own conclusions as to what is the best choice for patients they treat,” says co-author Bruce Strober, M.D., Ph.D. He is clinical professor of dermatology at Yale University School of Medicine and practices at Central Connecticut Dermatology in Cromwell, Conn.
“Over the past 18 years we’ve improved our ability to effectively clear skin in more people, and to make it easier on patients with regard to the frequency with which they dose themselves,” he says.
Furthermore, Dr. Strober adds, these advances in efficacy appear not to come at the expense of safety. And while these comments apply to entire biologic era, one could argue that drugs debuting over the past six years are even safer than the older biologics that provided less efficacy.
“Another important point is that we have multiple medications that treat psoriatic arthritis (PsA) well. We have a plethora of choices based on an increased understanding of the pathophysiology of psoriasis.”
Within the cornucopia resides complexity. “Not all TNF inhibitors, interleukin (IL)-17 inhibitors or IL-23 inhibitors are the same," he says. "Even within classes, there is a need for the provider to understand important distinctions in dosing frequency and efficacy.”
The IL-17 pathway inhibitors secukinumab, ixekizumab and brodalumab offer high efficacy for both the skin and PsA. “In many regards,” Dr. Strober says, “we should be viewing the IL-17 inhibitors as equal to or better than the TNF inhibitors. Specifically, ixekizumab and secukinumab treat the skin better than, and the joints as well as, TNF inhibitors.”
RELATED: Psoriasis guidelines emphasize patient education, comorbidities
The IL-23 inhibitors guselkumab, tildrakizumab and risankizumab are unique in their ability to clear skin effectively with fewer doses than their predecessors, yielding patient convenience.
“The one part of the IL-23 inhibitor story that is yet to be determined is how effectively they treat PsA," he says. "There’s no doubt most or all of them are going to gain approval for the treatment of PsA in the near future. It’s still unknown whether in this regard they are truly comparable to either TNF inhibitors or IL-17 inhibitors, specifically with regard to the inhibition of radiographic progression and the ability to treat axial psoriatic arthritis. There likely are differences between the drugs within the class.”
Also unclear, says Dr. Strober, is how long-term efficacy varies between biologics.
“If a patient starts a drug and is doing well on it after three months, what’s the probability he or she is still doing well on that drug a year later? There will be differences between various drugs, which will be uncovered through other types of studies that will be published or conducted in the near future,” he notes.
Real-world registry studies will be especially important, he says, because head-to-head clinical trials usually focus on only two drugs, and don’t last long enough (beyond a year) to provide a fully complete picture.
Comparison studies done to date generally show that IL-17 inhibitors, especially ixekizumab and brodalumab, work faster than TNF inhibitors and IL-23 inhibitors.
RELATED: Ixekizumab approved for pediatric plaque psoriasis
“But it’s possible that IL-23 inhibitors are more durable in the long run than IL-17 inhibitors,” Dr. Strober adds.
Ixekizumab (which blocks IL-17A) and brodalumab (which blocks the IL-17 receptor) provide unparalleled speed of response, with notable improvement and clearing of skin within two to four weeks. The pipeline IL-17A/F inhibitor bimekizumab, which UCB plans to submit for FDA approval in mid-2020, will be the fastest biologic drug ever, he says.
“The paradigm may continue to change, because bimekizumab will treat the joints and skin as well as or better than any drug, and faster than any drug.”
The only remaining questions, says Dr. Strober, are or whether it will be as safe and as long-lasting in its efficacy as previous biologics.
Disclosures:
Dr. Strober is a consultant (honoraria) for AbbVie, Almirall, Amgen, Arcutis, Arena, Aristea, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Janssen, Leo, Eli Lilly, Meiji Seika Pharma, Novartis, Pfizer, GlaxoSmithKline, UCB Pharma, Sun Pharma, Ortho Dermatologics, Regeneron and Sanofi Genzyme. He is also a speaker for AbbVie, Eli Lilly, Janssen and Ortho Dermatologics; an investigator for AbbVie, Corrona Psoriasis Registry, Dermavant and Dermira; and co-scientific director (consulting fee) of the Corrona Psoriasis Registry. He is the editor in chief of the Journal of Psoriasis and Psoriatic Arthritis.
References:
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.