- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Ixekizumab approved for pediatric plaque psoriasis
Author(s):
Eli Lilly announces the U.S. Food and Drug Administration approval of ixekizumab (Taltz) for treatment of moderate-to-severe plaque psoriasis in pediatric patients 6 and under 18 who are also eligible for phototherapy and systemic therapy.
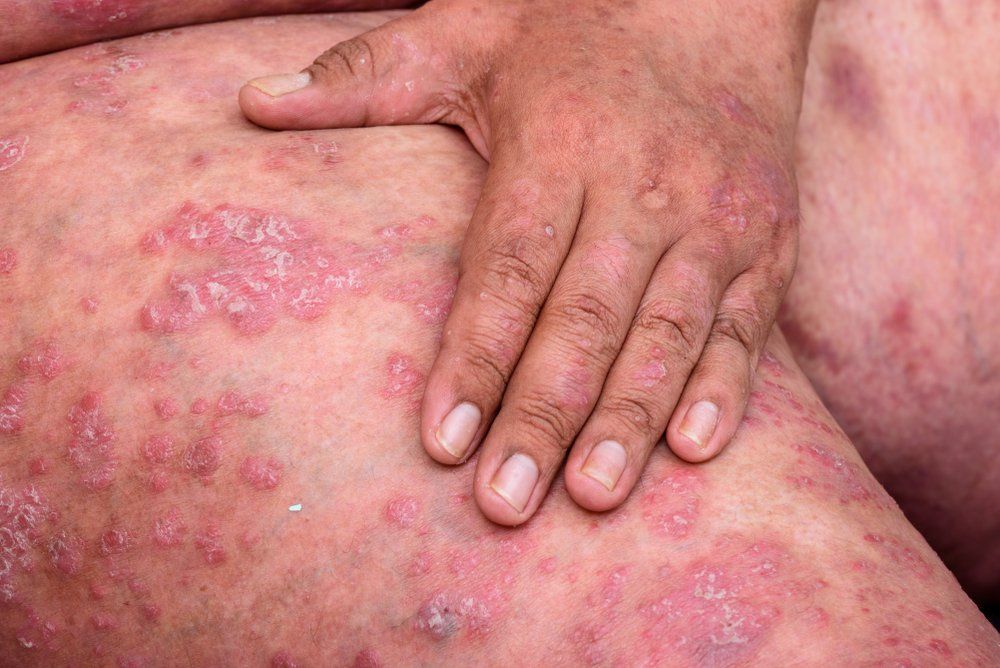
A new treatment for pediatric plaque psoriasis will soon be on the market after Eli Lilly announces they have received U.S. Food and Drug Administration approval of ixekizumab 80mg/mL (Taltz, Eli Lilly) for pediatric patients.
The drug’s supplemental Biologics License Application (sBLA) was approved March 30 for treatment of moderate-to-severe plaque psoriasis in pediatric patients ages 6 to under 18 who are also eligible for phototherapy or systematic therapy.1
MORE: Guidance on transitioning psoriasis patients between biologics
Ixekizumab is a monoclonal antibody that is designed to selectively bind with interleukin 17A (IL-17A) cytokine while inhibiting its interaction with the IL-17 receptor.
The approval announcement follows positive results of a phase 3, 12-week, double-blind, randomized, placebo-controlled clinical study investigating the efficacy, tolerability and safety of ixekizumab in 171 pediatric patients with moderate-to-severe plaque psoriasis.
During the trial, subjects were randomly given ixekizumab or placebo. For those who received the ixekizumab, dosage was based on weight with starting doses of 40 mg for <25 kg, 80 mg for 25-50 kg or 160 mg for >50 kg; those subjects then received 20 mg, 40 mg or 80 mg, respectively, for 12 weeks.
Co-primary endpoints include a proportion of subjects who achieve a static Physician’s global Assessment (sPGA) of clear or almost clear skin (0,1) and at least a 75% increase from baseline on the Psoriasis Area and Severity Index score (PASI 75) at week 12. Secondary endpoints were a proportion of subjects achieving sPGA (0), PASI 90, PASI 100 and reach a minimum of a four-point improvement in Itch Numeric Rating Scale (Itch NRS ≥4) at week 12. Other secondary endpoints included a proportion of patients reaching a PASI 75 and sPGA 0,1 at week 4.
Results of the study show the drug was superior to placebo with 89% of subjects who received ixekizumab reached PASI 75 versus 25% of placebo subjects. Additionally, 81% of ixekizumab patients achieved sPGA 0,1 versus 11% of placebo patients.
“In the phase 3 pediatric study, half of patients treated with Taltz achieved completely clear skin after only 12 weeks of treatment. These results and the subsequent FDA approval make a strong case for Taltz as an effective treatment option for doctors to consider for pediatric patients with moderate to severe plaque psoriasis," says Jennifer Cather, M.D., Modern Research Associates, Dallas, Tx.
The safety profile examined in the study was also consistent with previous safety profiles of ixekizumab except for the more frequent occurrences of influenza (2%), conjunctivitis (3%) and urticaria (2%). Also, this 12-week clinical trial exhibited higher incidences of Crohn’s disease in the ixekizumab group (0.9%) compared to the placebo group (0%).
Ixekizumab was initially FDA-approved in March 2016 for the treatment of moderate-to-severe plaque psoriasis in adults who are also eligible for phototherapy or systemic therapy. Since then, the drug has also been approved for the treatment of psoriatic arthritis in adults (December 2017) and ankylosing spondylitis in adults (August 2019).
RELATED: Psoriasis guidelines offer management pearls for pediatric patients
"We have over five years of data demonstrating that Taltz is a safe and effective treatment option for psoriasis in adults, and with this approval, we're pleased to now be able to offer Taltz to more people living with this challenging condition," says Patrik Jonsson, senior vice president and president of Lilly Bio-Medicines.
References:
Lilly's Taltz® (ixekizumab) Receives U.S. FDA Approval for the Treatment of Pediatric Patients with Moderate to Severe Plaque Psoriasis. Eli Lilly and Company. https://investor.lilly.com/news-releases/news-release-details/lillys-taltzr-ixekizumab-receives-us-fda-approval-treatment-1. Published March 30, 2020. Accessed April 8, 2020.






