- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Meta-analysis points to most effective biologics for psoriasis
Author(s):
Brodalumab, guselkumab, ixekizumab and risankizumab stood out among 15 biologic and oral medications as having the highest short- and long-term response rates for the treatment of moderate-to-severe plaque psoriasis, according to a recent meta-analysis.
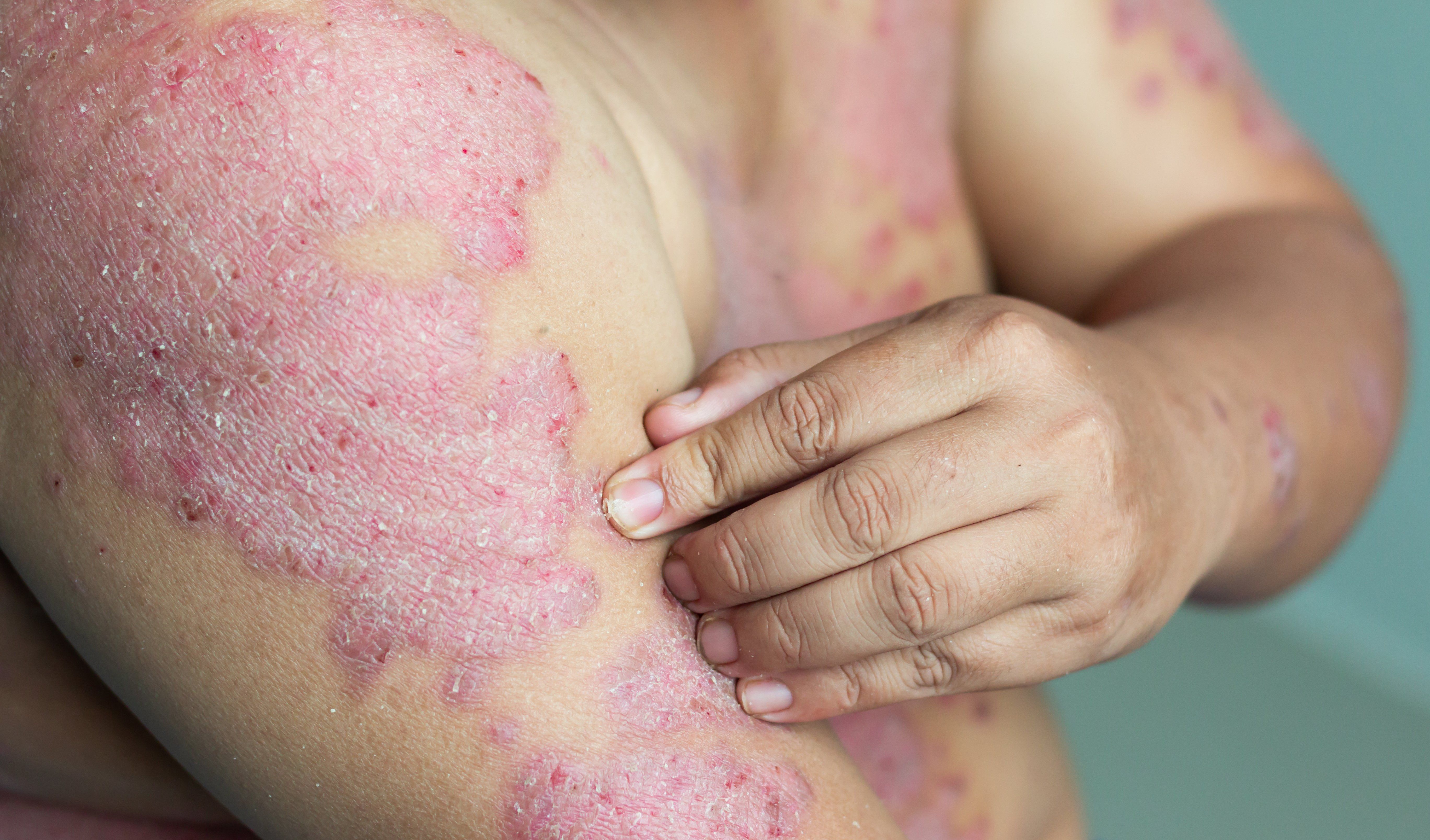
Brodalumab, guselkumab, ixekizumab and risankizumab stood out among 15 biologic and oral medications as having the highest short- and long-term response rates for the treatment of moderate-to-severe plaque psoriasis, according to a meta-analysis published February 5, 2020 in JAMA Dermatology.
READ MORE: Psoriasis guidelines emphasize patient education, comorbidities
“As dermatologists and dermatology providers, it can be confusing trying to decide among the various therapies that we have for psoriasis, especially at this time when we have a lot of different options,” said study author April W. Armstrong, M.D., M.P.H., a dermatologist with Keck Medicine of USC and professor of dermatology and associate dean for clinical research in dermatology at the Keck School of Medicine of USC. “In this study we performed a meta-analysis, where we took all the FDA and European Medicines Agency approved biologics and oral therapies and compared them in terms of their efficacy. We could do that because many trials that evaluate these medications are designed very similarly. We could take the clinical trial data from multiple medications and compare results of the trials as if they had a common placebo group, even though we don’t have head-to-head studies for all possible comparisons.”
Dr. Armstrong and colleagues studied data from 60 clinical trials, measuring Psoriasis Area and Severity Index (PASI) 75, 90 and 100 response rates at 10 to 16 weeks and 44 to 60 weeks from baseline.
They found risankizumab, brodalumab, ixekizumab, and guselkumab had the highest 10-to 16-week estimated PASI 90 response rate at 71.6%; 70.8%, 70.6%, and 67.3%, respectively.
Results at weeks 44 to 60 were similar with risankizumab having PASI 90 rates at 79.4%, guselkumab at 76.5%, brodalumab at 74% and ixekizumab at 73.9%.
“Findings were consistent for short-term and long-term PASI 75 and 100 responses,” the authors wrote.
RELATED: Anti-TNF therapy efficacy differs by gender
The researchers also studied secukinumab, infliximab, certolizumab pegol (400 mg), ustekinumab, adalimumab, certolizumab pegol (200 mg), tildrakizumab (200 mg), tildrakizumab (100 mg), etanercept, apremilast, dimethyl fumarate and placebo. For comparison, the estimated PASI 90 response rate at 10 to 16 weeks for apremilast was 12.1%; etanercept was 17.9%; adalimumab was 43.7%; ustekinumab was 43.9%, infliximab was 57.4% and secukinumab was 61.4%.
Practitioners can use this study to help compare efficacies among these systemic medications for psoriasis, according to Dr. Armstrong. However, she added, efficacy is only part of the picture. It’s also important to think about a medication’s safety record and its ability to treat psoriatic arthritis.
“They can reference this article because it talks about how these drugs compare in regards to efficacy, by a specific time and looking at specific outcomes. For example, if they want to know if biologic A is better than biologic B in terms of the proportion of patients that will get clear by week 60, they might look at this particular article and get a good sense of that,” she said. “One thing that we didn’t look at in this article is how do the different agents compare in terms of safety. The reason that cannot be done, typically, is because all of the studies are not powered for safety comparisons.”
The article speaks to the high and robust efficacies possible with some of today’s interleukin (IL) 17 and IL-23 medications, Dr. Armstrong said.
But it’s still important to individualize therapy, according to Dr. Armstrong.
“If patients have psoriatic arthritis, for example, that should be accounted for when you are selecting a medication because all of our IL-17 inhibitors are approved for psoriatic arthritis, whereas our IL-23 inhibitors at the current time are not yet approved for psoriatic arthritis,” she said.
Another consideration when individualizing therapy is patient comorbidities.
“If patients have demyelinating disease, they can’t be on a [tumor necrosis factor] TNF inhibitor. Or if they have a personal history of Crohns disease, then we probably aren’t going to put them on an IL-17 inhibitor,” Dr. Armstrong said. “You are still left with a number of choices, and I think this article will be informative with regards to answering the question: ‘If I want to give this patient the best efficacy, which drug would give the best chance of achieving that?’”
READ MORE: Anti-TNF therapies may not contribute to cancer risk
As for where that leaves others medications that did not quite rise to the top, Dr. Armstrong said that if patients are on those drugs and are doing well, there’s no reason to switch.
“Those drugs still have an important role for patients. The older drugs probably have longer record of safety, so that’s very important. A lot of the newer drugs have robust efficacy but they are relatively newer, so their long-term safety is not as established as the older drugs,” she said.
In addition to considering a drug’s safety, dermatologists have to think about access.
“Our older drugs may have better access for patients than the newer ones,” Dr. Armstrong said.
Authors of an accompanying editorial noted that network meta-analyses, like the one in the paper by Armstrong et al, rely on head-to-head comparisons within trials and indirect comparisons across trials.
“The strength of network meta-analyses is the ability to standardized drug effect across many trials,” the editorial authors wrote. “Indeed, the raw rankings of efficacy as determined by the Armstrong et al [network meta-analysis] and other recently published [network meta-analyses] offer a valuable starting point from which to initiate discussions with patients and improve shared decision making related to choosing the most appropriate psoriasis treatment.”
Disclosures:
Disclosures: Dr. Armstrong reported to JAMA that she received grants and personal fees from AbbVie, Eli Lilly, Leo Pharma, and Novartis Pharmaceuticals Corp; personal fees from Boehringer Ingelheim/Parexel, Bristol-Myers Squibb, Celgene, Dermavant, Janssen Pharmaceuticals Inc, Merck, Modernizing Medicine, Ortho Dermatologics, Pfizer Inc, Regeneron Pharmaceuticals, Sanofi Genzyme, Science 37 Inc, Genentech, GlaxoSmithKline, and Valeant; and grants from Dermira, Janssen-Ortho Inc, Kyowa Hakko Kirin, and UCB Pharma outside the submitted work.
AbbVie, which markets Skyrizi (risankizumab-rzaa) funded the research and participated in it, including in the interpretation of data.
References:
Armstrong AW, Puig L, Joshi A, et al. Comparison of Biologics and Oral Treatments for Plaque Psoriasis: A Meta-analysis. JAMA Dermatol. 2020;156(3):258–269. doi:10.1001/jamadermatol.2019.4029.






