- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
FDA grants priority review for dupilumab in pediatric AD
Author(s):
The FDA announced it has accepted a priority review of dupilumab for the treatment of moderate-to-severe AD in pediatric patients 6 to 11 years of age. The drug is already approved for treatment of AD in adolescents and adults.
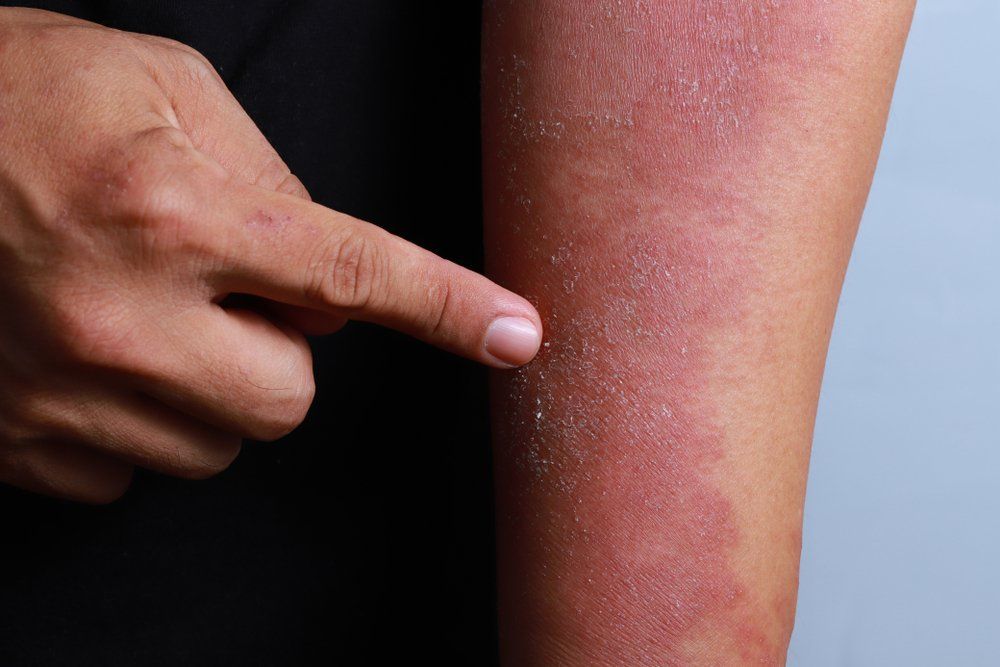
Another therapy for pediatric atopic dermatitis (AD) may be around the corner sooner than expected with the U.S. Food and Drug Administration (FDA) recently announcing it has accepted dupilumab (Dupixent; Sanofi and Regeneron Pharmaceuticals, Inc.) for priority review of its supplemental Biologics License Application (sBLA). The application is for the drug’s use to treat moderate-to-severe atopic dermatitis (AD) in pediatric patients 6 to 11 years of age when other prescribed topical therapies are not effective or recommended.
MORE: Dupilumab offers safe, long-term option for atopic dermatitis
Dupilumab is a monoclonal antibody that works to impede the signaling of IL-13 and IL-4 antibodies using Regeneron’s VelocImmune technology, which uses genetically humanized mice to yield human antibodies. IL-13 and IL-4 have been shown to play a key role in type 2 inflammation found in AD and other inflammatory conditions, according to a company press release.1
The decision to grant priority review by the FDA follows results of phase 3 clinical trials examining the efficacy and safety of dupilumab in combination with a topical corticosteroid (TCS) in pediatric patients with severe AD.
Results from the study showed when participants were given dupilumab and TCS, improvements were made in skin clearance, itching, disease severity and health-linked quality of life versus patients who were solely treated with TCS.
Some younger patients experienced adverse events including injection site reactions, conjunctivitis and nasopharyngitis, which coincides with similar previously recorded adverse events with dupilumab in older AD patients.
In 2016, the FDA granted dupilumab breakthrough therapy designation for treatment of severe AD in children 6 months to 11 years of age and moderate-to-severe AD in adolescents 12 to 17 years of age, and was later approved by the FDA for treatment of adolescents in March 2019.2
Currently in the U.S., dupilumab is approved to treat moderate-to-severe AD patients 12 years of age and older if topical therapies are not effective. The drug has also been approved in combination with other medications to treat asthma in patients 12 years of age and older and adults with chronic rhinosinusitis with nasal polyposis (CRSwNP).
RELATED: Real-world dupilumab use for adult atopic dermatitis
If the drug is approved for pediatric use, dupilumab could become the first biologic treatment in the U.S. for pediatric patients (6 to 11 years of age) whose conditions have persisted through topical prescription therapies.
The FDA is expected to make a decision on the application on May 26, 2020.
References:
1. Regeneron Pharmaceuticals, Inc. FDA Accepts for Priority Review Dupixent® (dupilumab) for Children Aged 6 to 11 Years with Moderate-to-Severe Atopic Dermatitis. PR Newswire: press release distribution, targeting, monitoring and marketing. https://www.prnewswire.com/news-releases/fda-accepts-for-priority-review-dupixent-dupilumab-for-children-aged-6-to-11-years-with-moderate-to-severe-atopic-dermatitis-300994046.html. Published January 28, 2020. Accessed January 30, 2020.
2. Sanofi. DA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents - Mar 11, 2019. Sanofi. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Published March 11, 2019. Accessed January 30, 2020.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.









