- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Real-world dupilumab use for adult atopic dermatitis
Author(s):
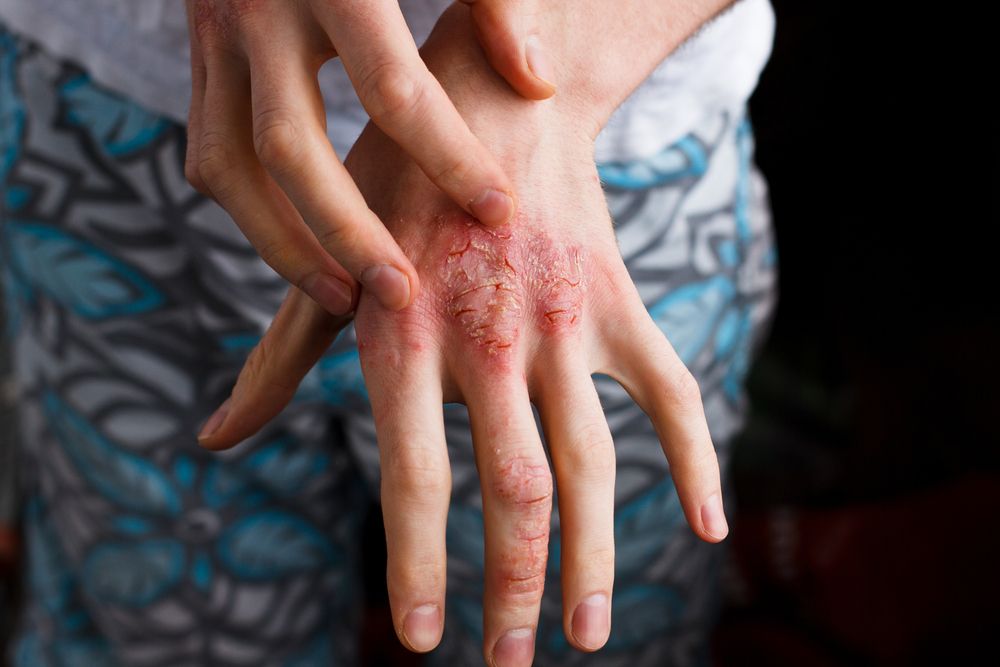
Real-world outcomes from treating adults with moderate-to-severe atopic dermatitis with dupilumab are similar to what researchers have found in clinical trials, according to this study.
“This study provides further information on our experience with dupilumab in moderate-to-severe atopic dermatitis and its efficacy and tolerability in our patient population, further supporting the use of this therapeutic modality,” says study authors Sergei Grando, M.D., Ph.D., D.Sc. and Christina N. Kraus. M.D. (©TernavskaiaOlgaAlibec/Shutterstock.com)
Dr. Grando

Dr. Kraus

Real-world outcomes from treating adults with moderate-to-severe atopic dermatitis with dupilumab (Dupixent, Sanofi and Regeneron Pharmaceuticals) are similar to what researchers have found in clinical trials. Dupilumab is generally well tolerated and the majority of patients on the drug experience clinical improvement-many benefit with complete clearance of atopic dermatitis, according to a recent report in the International Journal of Dermatology.1
RELATED: Dupilumab a game-changer in pediatric atopic dermatitis
“This study provides further information on our experience with dupilumab in moderate-to-severe atopic dermatitis and its efficacy and tolerability in our patient population, further supporting the use of this therapeutic modality,” according to an email response from two of the report’s authors Sergei Grando, M.D., Ph.D., D.Sc. and Christina N. Kraus. M.D. “Our results also suggest patients may require at least 16 weeks of treatment before clinical response can adequately be assessed. To the best of our knowledge, this is one of the first reports of real-world experience with dupilumab, and our findings are well in-line with reports from clinical trials.”
U.S. researchers retrospectively studied the medical records of 77 men and women with atopic dermatitis who were treated with dupilumab in the University of California Irvine’s dermatology department. The records from May 1, 2017 to January 25, 2019 included patients who received standard dosing, which is an initial dupilumab dose of 600 mg, followed by injections every other week of 300 mg each.
Dupilumab, FDA-approved in March 2017 to treat moderate-to-severe atopic dermatitis in adults, is a monoclonal antibody against the interleukin-4 receptor. Clinical trials have shown dupilumab is safe and effective as monotherapy or in conjunction with other atopic dermatitis therapies. All 77 patients had a documented follow-up visit within 16 weeks of starting dupilumab treatment and all had received at least one medication dose. All had tried other treatments for their atopic dermatitis.
There have been two other reports of clinicians’ real-world experiences using the drug, including retrospective multicenter studies in Spain2 and in France3.
The researchers in Spain looked at dupilumab treatment outcomes in 27 patients with at least 12 weeks of follow-up, noting improvements in atopic dermatitis activity and pruritis intensity. French researchers studied 241 patients and found a significant reduction in atopic dermatitis severity at three months. More than 38% of patients in that study reported conjunctivitis during treatment.
RELATED: Safety of systemic immunomodulatory drugs in atopic dermatitis
U.S. AUTHOR FOUND:
Thirty-percent (or 23 patients) were in complete clinical remission with dupilumab treatment. Doctors encouraged two of those patients to taper off dupilumab after a year of treatment. One is in stable remission at least six weeks after discontinuing dupilumab, the authors report.
Fifty-six percent (43 patients) experienced partial but stable improvement in atopic dermatitis.
And 14% (11 patients) had no improvement or worsening atopic dermatitis severity.
Among the patients studied, 73 used topical or systemic medications while on dupilumab. While most used topical corticosteroids in addition to dupilumab, others were treated concomitantly with crisaborole, intramuscular systemic corticosteroids, UV light therapy and topical calcineurin inhibitors.
More than a quarter of patients reported adverse events during treatment. Of the 20 patients who did, dry eyes was most common, followed by conjunctivitis, keratitis, blurry vision, herpes simplex virus infection and blepharitis. One patient experienced an ophthalmic foreign body sensation and one experienced hypersensitivity. The treating physician stopped dupilumab in the patient with hypersensitivity after the hypersensitivity recurred upon re-challenge, according to the report.
“A majority of cases of ophthalmologic involvement as a reported side effect improved after initiation of appropriate therapy by ophthalmology,” the authors write.
Real-world results suggest dupilumab is an excellent treatment modality for moderate-to-severe atopic dermatitis, as it is effective and better tolerated than other immunomodulators used in patients with atopic dermatitis, Drs. Grando, professor of dermatology and biological chemistry, and Kraus, resident physician in dermatology at University of California, Irvine, write.
RELATED: Diagnosing adult-onset atopic dermatitis
“Overall, what we can conclude from our study is that the majority of patients do very well on this medication,” they write. “Of the eleven patients that did not improve while on dupilumab, ten had been on the medication for less than 16 weeks (and one was misdiagnosed). While many of our patients experienced clinical improvement rapidly, these findings suggest there may be a subset of patients that require a longer duration of initial treatment prior to responding and thus, the medication may have been discontinued too soon to adequately assess response. The patients with the most severe cases of atopic dermatitis had the longest delay in achieving therapeutic effect.”
The remaining symptoms can be safely managed by co-administration of various topical agents, anti-histamines, systemic steroids or light therapy, according to Drs. Grando and Kraus.
Disclosures:
None
References:
1. Wang C, Kraus CN, Patel KG, Ganesan AK, Grando SA. Real-world experience of dupilumab treatment for atopic dermatitis in adults: a retrospective analysis of patients' records. Int J Dermatol. 2019;
2. Ruiz-villaverde R, Dominguez-cruz J, Armario-hita JC, Martinez-pilar L, Alcantara-luna S, Pereyra-rodriguez JJ. Dupilumab: short-term effectiveness and security in real clinical practice - A retrospective multicentric study. J Eur Acad Dermatol Venereol. 2019;33(1):e21-e22.
3. Faiz S, Giovannelli J, Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019;81(1):143-151.






