- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Dupilumab offers safe, long-term option for atopic dermatitis
Author(s):
A recent study reports findings that support dupilumab as a safe, long-term treatment in patients with moderate-to-severe atopic dermatitis.
This is the first study to evaluate safety in patients receiving dupilumab treatment for more than 52 weeks, write the authors. (©TernavskaiaOlga/Shutterstock.com)
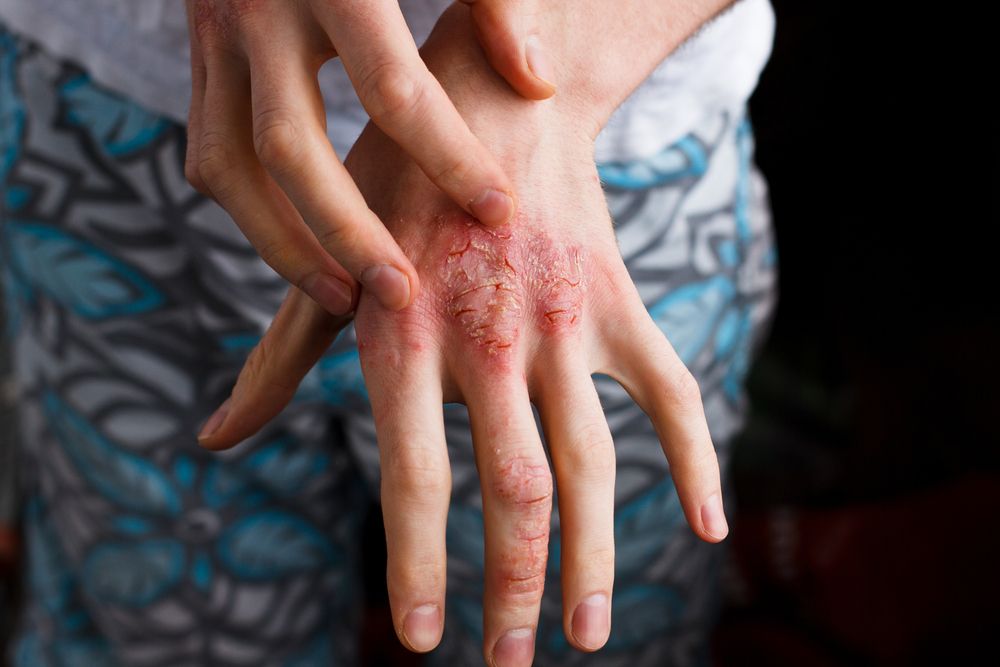
A study in Journal of the American Academy of Dermatologyreports findings that support dupilumab as a safe long-term treatment in patients with moderate-to-severe atopic dermatitis (AD).
RELATED: New ways to tackle itch in AD
The phase 3, multicenter, open-label extension study examined the long-term treatment of AD with dupilumab in adults (18 years and older) who had previously participated in phase 1, 2 and 3 clinical trials for the drug. The study’s primary endpoint was long-term safety, evaluated as incidence and rate of adverse events (AEs).
A total of 1491 patients from North America, Europe and the Asia-Pacific region participated in the study. Exclusion criteria included AEs related to dupilumab and serious AEs (SAEs) that led to discontinuing dupilumab treatment in previous clinical trials.
Patients received a dupilumab loading dose of 600 mg, then 300 mg weekly, or a loading dose of 400 mg, then 200 mg weekly, before the protocol was amended to 300 mg weekly. Data was collected for up to 76 weeks of treatment. Most patients (98.6%) were at least 80% adherent to treatment.
According to the authors, 4384 AEs were reported. Many patients (70.7%) had at least one AE. Few patients (5%) had one or more SAEs. The most common AEs included conjunctivitis, nasopharyngitis, upper respiratory tract infection, AD and headache. Study drug-related SAEs included Hodgkin disease, prostate cancer, enterocolitis, serum sickness, eczema herpeticum, herpes ophthalmic, epilepsy and eczema. There were no deaths reported.
This is the first study to evaluate safety in patients receiving dupilumab treatment for more than 52 weeks, write the authors. While previous studies of the immunosuppressive agent’s cyclosporine and azathioprine have experienced limitations due to high rates of patient discontinuation, this study had a very low (1.8%) discontinuation rate.
RELATED: AD pipeline full of agents in various phases of development
The study results are consistent with findings of other studies analyzing the safety and efficacy of dupilumab, according to the authors. Efficacy was sustained for the treatment period of 76 weeks, thereby improving QoL, study authors report. Less than half of patients used additional treatment for AD during the study.
“This, combined with the relatively small numbers of patients requiring systemic rescue therapy, supports the idea that dupilumab monotherapy or concomitant with topical AD medications provides long-term disease control in patients with moderate to severe AD,” they write.
Authors note limitations to the study include the proportion of patients that were able to reach 76 weeks of treatment and a lack of a control arm but add that the results suggest the benefits of this long-term treatment.
RELATED: Study investigates real-world dupilumab use in adult AD
“The favorable benefit-risk profile in this study supports the long-term role of dupilumab treatment for patients with moderate-to-severe AD and shows that blocking IL-4 and IL-13 signaling can achieve sustained control of AD signs and symptoms with an acceptable safety profile in patients with significant disease burden and for whom conventional topical treatments are inadequate,” they write.
References:
Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2019;







