- Case-Based Roundtable
- General Dermatology
- Eczema
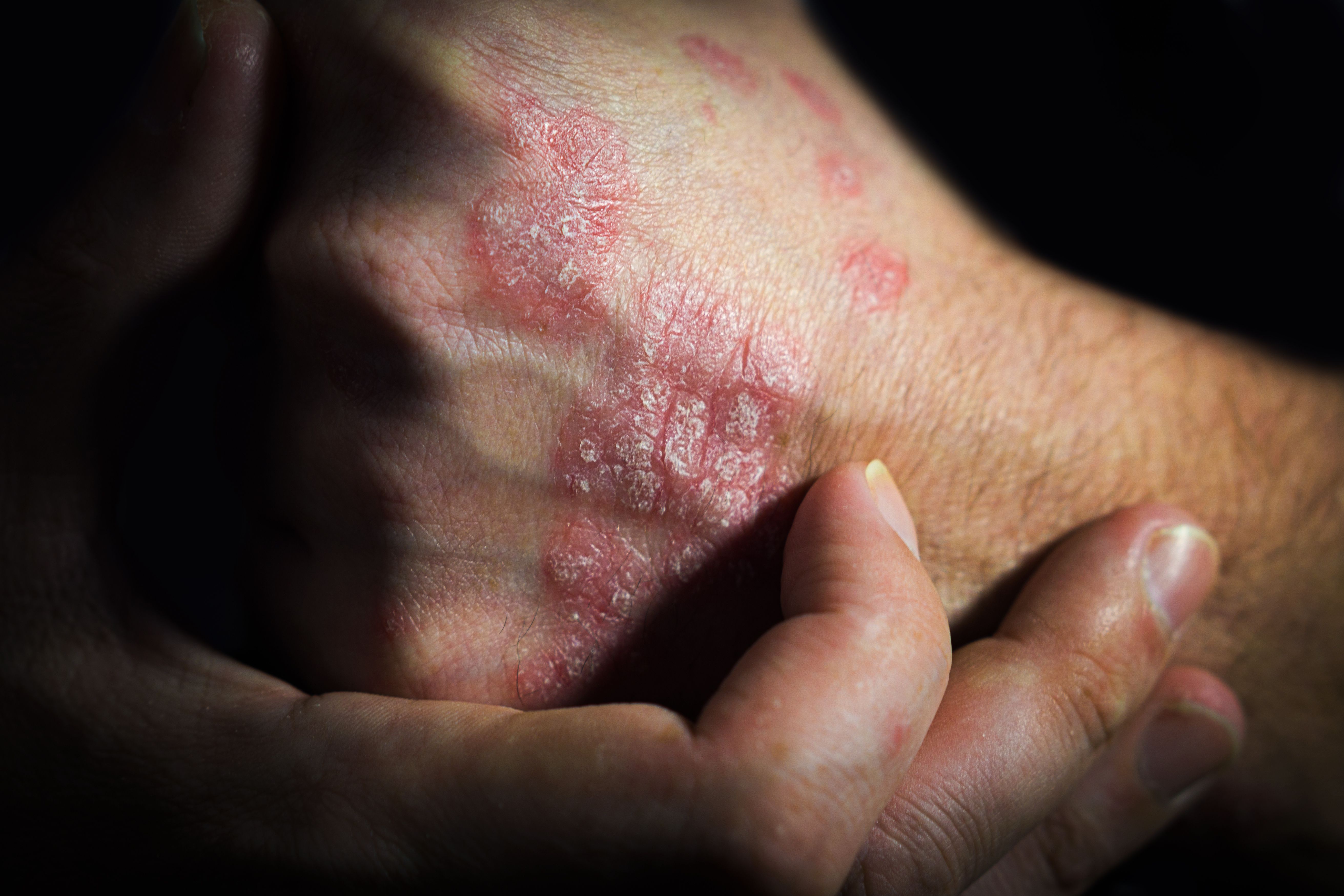
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
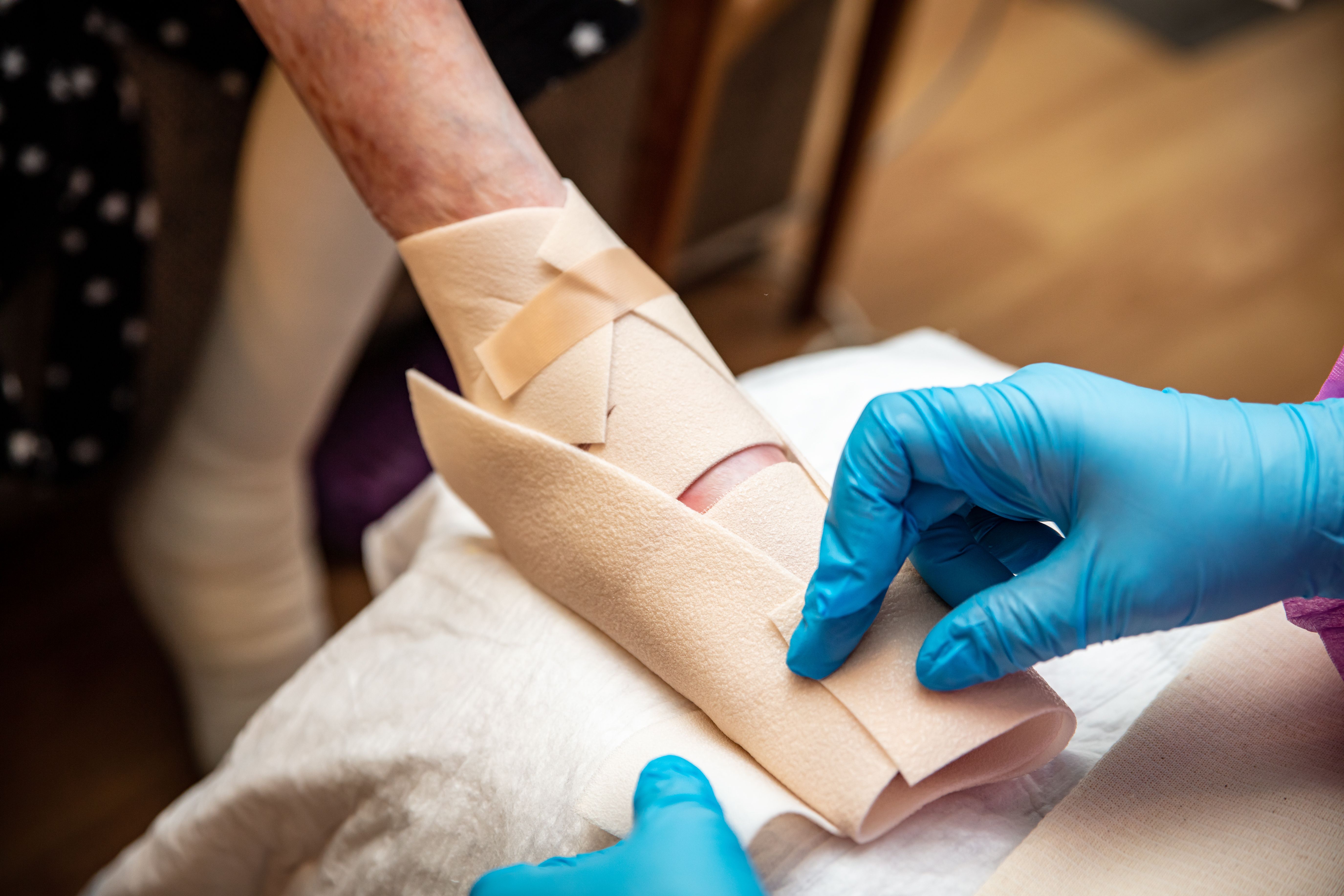
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Video
What are Biologics and Biosimilars, and How Are They Devolved?
Roy M. Fleischmann, MD and Joel M. Gelfand, MD, MSCE, FAAD define the terms biologic and biosimilars and discuss how they are developed.
Roy M. Fleischmann, MD: Hello, and thank you for joining this Dermatology Times® DermView, “A Game-Changer in Chronic Inflammation Management.” I’m Dr Roy Fleishmann, a clinical professor of medicine at the University of Texas Southwestern Medical Center, and co-medical director of Metroplex Clinical Research Center in Dallas, Texas. I’m joined by Dr Joel Gelfand, the vice chair of clinical research, the medical director of the dermatology clinical studies unit, the director for the psoriasis and phototherapy treatment center, and a professor of epidemiology in biostatistics and epidemiology at the University of Pennsylvania School of Medicine in Philadelphia, Pennsylvania. He’s also the James J. Leyden, MD, Endowed Professor in Clinical Investigation.
In this discussion, we’ll highlight the regulatory process to approve a biosimilar, highlight the use and impact of interchangeable biosimilars, and discuss how biosimilars can alter the treatment landscape in chronic conditions. Let’s begin.
Let’s start with a discussion. What is a biologic, and why are they very complex to manufacture? Unlike traditional DMARDs [disease-modifying antirheumatic drugs], which are small-molecule chemicals, biologics are produced by biotechnology and are genetically engineered to act like natural proteins in the immune system. Because they are proteins, they must be delivered by intravenous infusion or injection. If taken orally, they’ll be digested in the gastric system. They are highly specific and target a specific pathway of the immune system. Some may be monoclonal, chimeric, or humanized fusion antibodies, whereas others are receptors that have been fused to part of the immunoglobulin.
How is a biosimilar defined? A biosimilar is a biological product that’s approved based on the totality of evidence demonstrating that it’s designed similar to the approved biological product, or the originator or reference product, in terms of structure, function, quality, purity, potency, clinical efficacy, and safety.
What is the regulatory process or requirements to create a biosimilar? How is a biosimilarity study conducted? For the FDA and the EMA [European Medicines Agency], the goal of a biosimilar development program is to demonstrate biosimilarity between the proposed biosimilar product and the reference product, not to independently establish the safety and effectiveness of the proposed product. The manufacturing proposed by a similar product generates an array of data comparing the proposed product to the FDA-approved reference product to demonstrate biosimilarity. Comparative data are generated and evaluated in a stepwise fashion that begins the foundation with detailed analytical, structural, and functional characterizations and comparison of the products, moving on to animal studies if necessary and then to comparative clinical studies.
Consequently, rather than generating the same full profile of nonclinical and clinical data as the reference product, a manufacturer that shows its proposed biosimilar product is highly similar to and has no clinically meaningful difference from the FDA-approved reference product may rely in part on the FDA’s previous determination of safety and effectiveness for the reference product for approval. Joel, what does this mean in English to the practicing rheumatologist or dermatologist?
Joel M. Gelfand, MD, MSCE, FAAD: Roy, that’s a nice overview of a complicated topic. The way clinicians need to think about it is that essentially a biosimilar product is just that. It isn’t identical, but the variation in the protein structure or glycosylation isn’t meaningfully different in any way. The chemical analysis done basically shows that any variation isn’t in the critical domains of where the biologics target the molecule, such as TNF [tumor necrosis factor].
There are a variety of ways that these therapies are studied. We could discuss that in more detail. Some studies look at only 1 disease. It might be only RA [rheumatoid arthritis]. Other studies may look at a multitude of diseases, such as psoriasis, RA, and inflammatory bowel disease. The agency is looking for a margin of what they say is noninferiority. Usually it’s about 15%, that the patients get the same outcome within 15% of one another. That would be a similar enough outcome. One biosimilar product shouldn’t necessarily have a better or worse outcome than the originator product.
Roy M. Fleischmann, MD: The FDA sets regulations to demonstrate biosimilarity. The biosimilar product application must include data demonstrating biosimilarity to the reference product, which usually includes data from the analytical studies, and as Joel said, demonstrated biologic properties similar to the reference product, notwithstanding minor difference in clinically inactive components. Then there are animal studies, including assessment of toxicity. Then, as Joel said, it could be a clinical study or studies sufficient to demonstrate the safety, purity, and potency of the proposed biosimilar product in 1 or more of the indications for which the reference product is licensed. This also typically includes assessing immunogenicity, pharmacokinetics, and in some cases pharmacodynamics. It also usually includes a comparative clinical study.
Transcript Edited for Clarity