- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Treatment options for acne expand
Author(s):
Read more on the variety of new treatment options for acne, with even more awaiting approval.
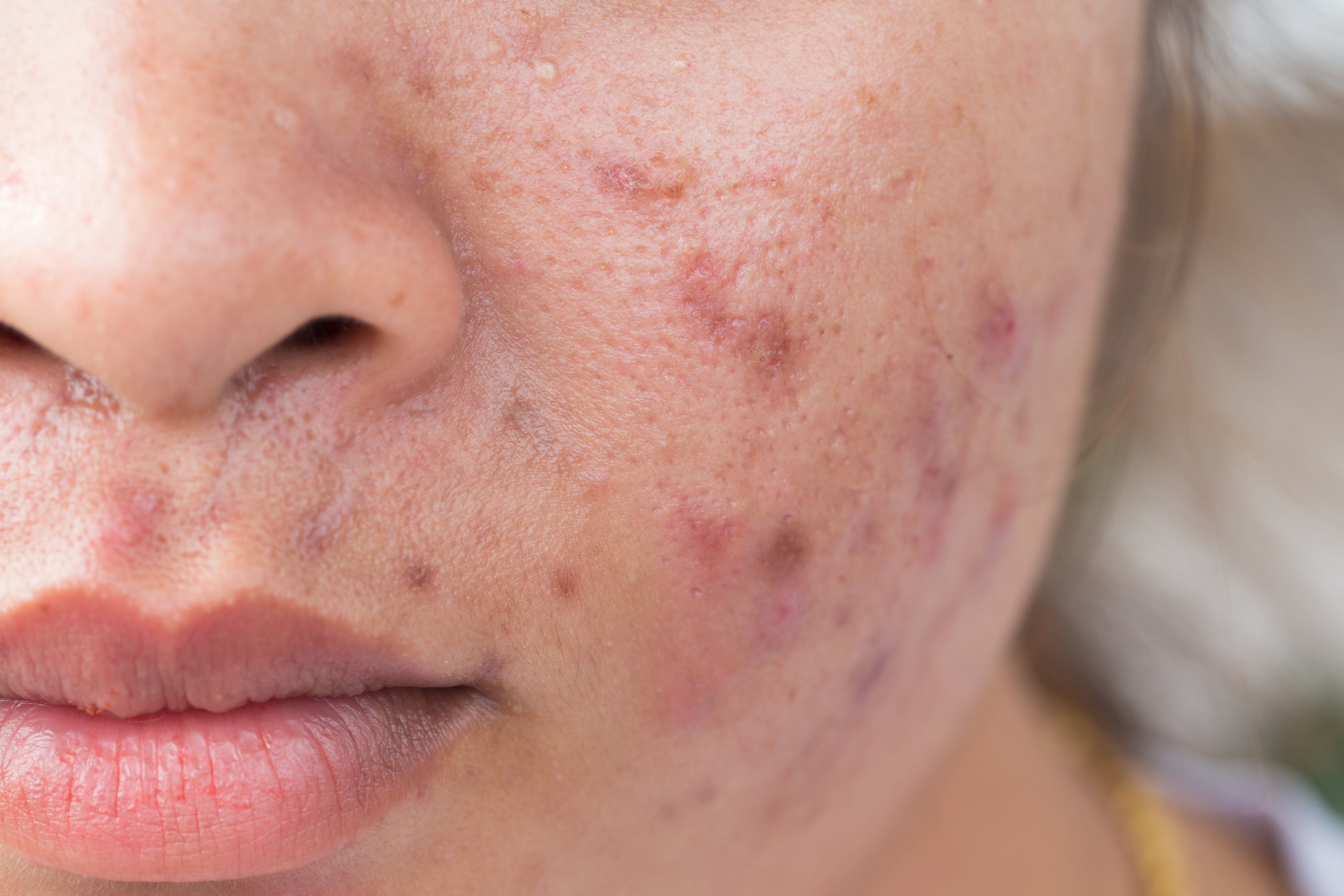
Options for medical acne treatment have expanded significantly since October 2018 with the U.S. Food and Drug Administration (FDA) approval of several novel products, and the future may hold more developments, says Lawrence F. Eichenfield, M.D., who spoke recently at the Maui Derm for Dermatologists 2020 meeting, Maui, Hawaii.
MORE: An update on antibiotic use for acne
“It has been a busy time in acne drug development leading to the availability of new topical and oral products across the spectrum of medication categories used to treat acne,” says Dr. Eichenfield, professor of dermatology and pediatrics, University of California, San Diego, and chief, pediatric and adolescent dermatology, UCSD/Rady Children’s Hospital, San
Diego.
RETINOIDS
Trifarotene cream 0.005% (Aklief, Galderma) is a novel retinoid approved by the FDA in October 2019 for the topical treatment of acne vulgaris in patients aged 9 years and older. Trifarotene is distinguished from tretinoin and adapalene pharmacologically by having >20-fold selectivity for the gamma subtype of the retinoic acid receptor versus RARα and RARβ.
Efficacy and safety of the 0.005% cream formulation were established in two phase 3 studies where it was compared with vehicle for treatment of moderate acne on both the face and the trunk. At the end of the 12-week double-blind treatment period, trifarotene demonstrated statistical superiority to vehicle in all primary and secondary efficacy endpoints that analyzed investigator global assessment and patient global assessment success rates and changes in inflammatory and non-inflammatory lesions.
“Trifarotene is the first topical retinoid studied for treatment of acne on the trunk, an area for which there was historically reluctance to use a topical retinoid due to concerns about lack of efficacy and the potential for excessive systemic exposure,” says Dr. Eichenfield.
“Results from a long-term extension study conducted primarily to evaluate safety showed there was a continuous improvement in the percentage of patients achieving both IGA and PGA success over time. At week 52, 58% of patients achieved overall success defined as achieving both IGA and PGA success, and there was no observed fall of responsiveness over time.”
Topical trifarotene was very well tolerated. Adverse event data showed erythema, scaling, dryness, stinging and burning were generally mild, occurred at low rates and dissipated with ongoing treatment.
Tazarotene lotion 0.045% (Arazlo, Ortho Dermatologics) was approved by the FDA for the treatment of acne vulgaris in patients aged 9 years and older in December 2019. It formulates the retinoid in a novel vehicle at a lower concentration compared with the gel product that is approved for acne.
“Tazarotene is a very potent topical retinoid, but it has been used inconsistently for the treatment of acne because it can be quite irritating,” says Dr. Eichenfield.
“The lotion formulation of tazarotene is a polymeric emulsion technology in which the active and emollient ingredients form an oil-in-water emulsion that is structured by a three-dimensional mesh matrix. The technology enables rapid and even ingredient release.”
The phase 3 studies of the new tazarotene product enrolled patients with moderate or severe acne aged 9 years and older. The results showed statistically significant differences favoring the tazarotene lotion versus vehicle at week 12 in the percentage of patients achieving a two-grade improvement from baseline with a rating of clear or almost clear disease and in analyses of percent change in both inflammatory and non-inflammatory lesions.
Severity of scaling, erythema, itching, burning and stinging were rated on a scale of 0 (none) to 3 (severe) and the mean scores for all of those adverse events were close to 0.
ANTIBIOTIC ALTERNATIVES
Recently approved antibiotics for the treatment of acne include both topical and oral products. The efficacy and safety of minocycline foam 4% (Amzeeq, Foamix), which was approved for the treatment of inflammatory lesions of non-nodular moderate-to-severe acne vulgaris in October 2019, was investigated after extensive dermal safety studies were completed that showed no evidence of phototoxicity, photoallergy, skin sensitization, or clinically significant cumulative skin irritation. In addition, a pharmacokinetics study was done that showed very low systemic absorption with topical application for 21 days.
The phase 3 studies enrolled patients aged 9 years and older with moderate-to-severe acne who were randomized to once daily treatment with vehicle or minocycline foam 4%. They met their co-primary efficacy endpoint that analyzed IGA treatment success and absolute change from baseline in inflammatory lesion count at week 12.
In addition, a subgroup analysis of data from three randomized, double-blind phase 3 studies showed that the efficacy of minocycline foam 4% in pediatric patients (ages 9 to 17 years) was similar to that seen in the overall study population. An open-label pediatric study investigating treatment under maximum-use conditions showed that minocycline foam 4% was generally safe, well-tolerated and associated with low minocycline systemic exposure.
RELATED: Expert offers insight on isotretinoin use
Sarecycline (Seysara, Almirall) is an oral tetracycline-derived antibiotic approved in October 2018 for once daily treatment of moderate-to-severe acne vulgaris in patients aged 9 years and older. Dosing is determined by patient weight and the clinical trials showed efficacy for treating both facial and truncal acne.
“In addition to its once daily dosing, sarecycline is distinguished from other tetracyclines by having minimal impact on Gram-negative enteric bacteria, although whether that difference has clinically relevant implications remains to be seen,” says Dr. Eichenfield.
AWAITING APPROVAL
The New Drug Application for clascoterone cream 1% (Winlevi, Cassiopea) as a treatment for acne is undergoing FDA review. Its mechanism of action for acne treatment is thought to be competitive inhibition of dihydrotestosterone binding to androgen receptors within sebocytes and hair papilla cells.
Topline results from the phase 3 studies enrolling patients with moderate-to-severe facial acne aged 9 years and older show the primary endpoints were met. Data from a long-term extension study showed that after 9 months, approximately 30% of patients achieved clear or almost clear ratings. Erythema and scaling, which tended to decrease over time, were the most common side effects.
“Importantly, there was no evidence of adverse effects on hair production and no evidence of absorption resulting in systemic hormonal effects,” says Dr. Eichenfield.
MORE UPDATES
Dr. Eichenfield also reminded attendees about the publication of evidence-based recommendations for the management of spontaneous and isotretinoin-induced acne fulminans.1
“There is limited data in the literature from prospective studies on this severe variant of inflammatory acne, and there have not been any clear guidelines about its treatment,” he says. “These guidelines were developed based on a systematic literature review and expert consensus to help practitioners classify acne fulminans and to treat it with oral steroids and isotretinoin when appropriate.”
Looking ahead, ongoing research focusing on the pathophysiologic importance of different strains of Cutibacterium acnes and Staphylococcus epidermidis may lead to new skin microbiome-based treatments for acne, including vaccines. New insights on acne pathogenesis that may identify future therapeutic targets may also come from research that is analyzing cytokine and gene expression profiles.
Disclosures:
Dr. Eichenfield is an investigator and/or consultant for Allergan, Almirall, Cassio- pea, Foamix, Galderma, L’Oreal, and Ortho Dermatologics.
References:
1 Greywal T, Zaenglein AL, Baldwin HE, et al. J Am Acad Dermatol. 2017;77(1):109-117.



























