- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Systemic Treatment for Pediatric AD
Author(s):
New and forthcoming options bring opportunity for optimizing patient care.
One-third of pediatric patients with atopic dermatitis (AD) have moderate to severe disease, and the affected proportion increases with age. While systemic medications should be considered for these patients when optimal therapy using basic management strategies and topical agents fails, safety concerns with systemic medications have limited their appropriate use.
The current and future availability of more targeted therapy for AD, however, promises to lower the threshold for starting systemic therapy and improving quality of life for pediatric patients with moderate to severe disease, says Amy S. Paller, MD, Walter J. Hamlin professor and chair of the Department of Dermatology and professor of pediatrics at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
“With its safety and lack of need for laboratory monitoring, dupilumab has revolutionized systemic treatment for moderate to severe AD in pediatric patients aged 6 years and older, and we can expect more advances in the future. Dupilumab is now being studied in children as young as 6 months of age, and pediatric trials are ongoing evaluating other biologics as well as oral Janus kinase (JAK) inhibitors,” Paller said.
She added: “In the future, identification of biomarkers, including those that can obtained noninvasively by tape stripping, may help predict the course of AD and its response to systemic medications and improve our therapeutic decision-making capabilities. In addition, pediatric-specific guidelines on AD reflecting new data are being developed to provide evidence-based recommendations. I expect these guidelines will be available within the next year.”
The algorithm for treatment of moderate to severe AD follows a stepwise approach that begins with topical anti-inflammatory medications and basic management strategies. If the AD is not responding, consideration should be given to nonadherence, misdiagnosis, the need to treat a secondary infection, or presence of a contact allergy or another allergic trigger, as well as intensity of topical treatment.
“We always want to ensure that topical therapy is optimized before considering commitment to a biologic,” Paller told Dermatology Times®. “I have seen many children with moderate to severe AD who dramatically improved with appropriate topical therapy. But there are risks associated with using potent topical steroids, especially if being applied in more sensitive areas. If topical steroids, often coupled with nonsteroidal topical alternatives, do not turn the situation around, dupilumab may be considered to offer a safer option than chronic application using large amounts of a topical steroid, especially with only partial improvement.”
When systemic therapy becomes indicated for AD in pediatric patients, the traditional options have been cyclosporine A, methotrexate, mycophenolate mofetil, and azathioprine.
“Outside the realm of pediatric dermatologists, treatment with systemic steroids remains the most commonly used approach, especially by primary care physicians. However, systemic steroids are too toxic for continued use in pediatric patients and AD rebounds once steroids are discontinued, so that use of systemic steroids in children with AD should be extremely restricted,” Paller said.
Cyclosporine has been the favored systemic treatment for AD among pediatric dermatologists because of its rapid onset and it remains the first option for children who cannot be on dupilumab (Dupixent; Sanofi Genzyme/Regeneron Pharmaceuticals) for any reason. However, cyclosporine is generally used for just 3 to 4 months to achieve control and then patients are transitioned to a less toxic agent, such as methotrexate, using an overlap period.
Offering advice for starting dupilumab, Paller suggested giving any required live immunizations at least 1 month before starting the biologic.
“It is not known that receiving a live immunization while on the biologic is unsafe and theoretically it is not, because dupilumab only blocks the Th2 signaling pathway. Nevertheless, the safety of live immunization while taking dupilumab has not been formally tested,” Paller said.
She called attention to the recommended age- and weight-based dosing schemes for dupilumab and cautioned against abruptly stopping any existing systemic treatment. With regard to the latter, Paller shared her practice of continuing any existing systemic agent (unless there is an adverse event) for 1 month and then reducing the dose by half for 1 month before discontinuation.
Topical steroids should be continued during the transition and until no longer needed to augment the effects of dupilumab. More potent topical steroids can be added for a short course as well to manage flares that may occur while the patient is on dupilumab, she said.
Excoriated erythematous papules on the antecubital area of a 10-year-old boy with severe atopic dermatitis. Photo courtesy of Amy Paller, MD.
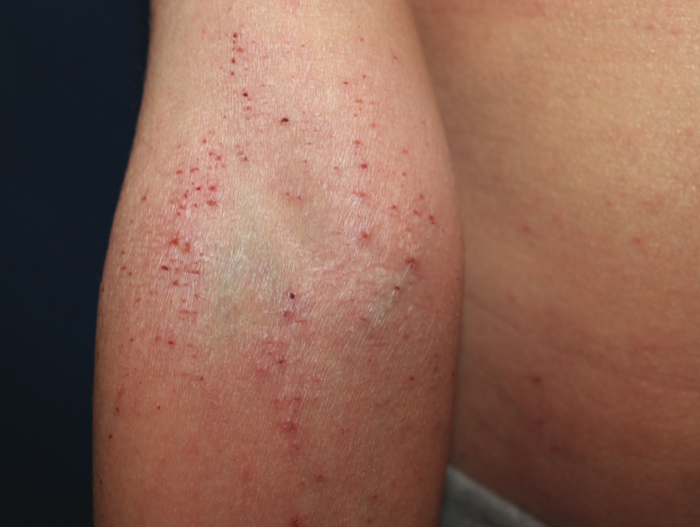
Not all patients respond to dupilumab, and while evidence of improvement is usually seen by 2 months in those who are likely to benefit from the biologic, dupilumab should be continued for at least 3 to 4 months before it is deemed ineffective.
“We can see improvement in some children beyond 16 weeks. For those without adequate improvement, however, by 3 to 4 months, increasing the dose or frequency of administration might help if insurance allows access to the higher dosing,” Paller said.
When treatment is effective, Paller suggested continuing dupilumab for at least 1 year of disease control. But if the patient or family is interested in trying to stop the dupilumab, it should never be stopped abruptly. Rather, she advised tapering administration by increasing the interval between injections while monitoring for relapse.
“Some children have sustained improvement when taken off dupilumab, suggesting that in some, the biologic may ‘reset’ the immune system, at least for several months. However, a patient whose AD flares during a dupilumab taper or who is not clear on the full dose of dupilumab will likely not do well if the treatment is stopped completely,” she said.
Pretreating with a topical anesthetic can be considered when a child is battling the dupilumab injection and it may be possible to eliminate the topical anesthetic after using it a few times as patients gain comfort. The pen form of dupilumab is useful for giving older children the independence to treat themselves, Paller said. However, she has found that it can be more uncomfortable for younger children, especially those with less subcutaneous tissue.
Conjunctivitis, which is the second most common adverse reaction with dupilumab, is not a contraindication to using the biologic, and if it emerges during treatment, the conjunctivitis is generally self-limiting after a few months.
Paller said that in her experience, starting use of preservative-free artificial tears when starting the dupilumab and continuing for the first few months may be helpful in children in reducing risk of conjunctivitis, but has yet to be tested. She recommended referring patients to an ophthalmologist for treatment of conjunctivitis that exists or if needed to control a reaction to dupilumab. She also mentioned a suggestion in the literature to apply tacrolimus ointment to the eyelids, especially if the eyelids are affected by AD, which they often are when conjunctivitis develops.
When discussing initiation of dupilumab with families, she encourages families to contact her if any unusual side effects of dupilumab occur, given the possibility of persistent red face, psoriasis/psoriasiform reactions, and new onset of alopecia areata.
“Starting dupilumab in a patient with comorbid alopecia areata would involve shared decision-making with the family and child, taking into account that there are also reports of the hair loss improving in children treated with dupilumab for AD and that the AD tends to be the greater burden,” Paller said.
Pregnancy outcomes in patients exposed to dupilumab while pregnant are being monitored. To date there is no evidence of risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes, but topical treatment is largely preferred during pregnancy, Paller said.
What’s in the pipeline
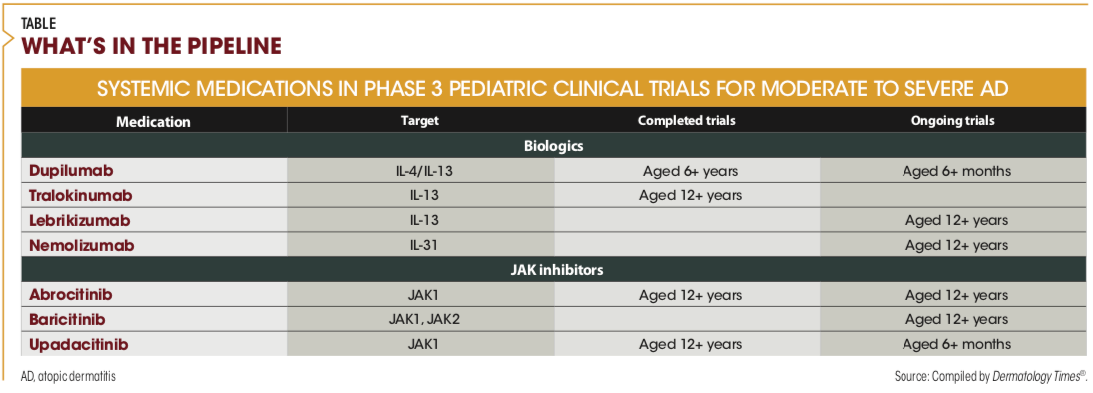
Two interleukin (IL)-13 inhibitors are being developed as treatment for AD in children and/or adolescents. Tralokinumab (LEO Pharma) has completed studies enrolling adolescent patients and is being investigated in a trial of children aged 6 years and above. Lebrikizumab (LY3650150; Eli Lilly and Company/Dermira, Inc) is being evaluated in ongoing trials including children aged 12 years and older. Studies to date suggest the same safety profile as dupilumab.
Nemolizumab (CD14152; Galderma), a monoclonal antibody targeting the receptor of IL-31, is now being studied in pediatric patients aged 12 years and older.
“IL-31 is itch-specific cytokine, and in studies of adults, nemolizumab had an early and dramatic effect on itch. It appears, however, that nemolizumab should be coupled with a topical corticosteroid to achieve the best effect on skin inflammation,” Paller said.
Three oral JAKs are also being investigated as treatment for AD in pediatric patients: abrocitinib (PF-04965842; Pfizer), which has completed a trial in patients aged 12 years and above; upadacitinib (Rinvoq; AbbVie Inc), which has completed a trial in the same age group and is now being studied in patients aged 6 months and above; and baricitinib (Olumiant; Eli Lilly and Incyte), which is in a study of adolescents aged 12 years and older.
Paller said available data from studies of the JAK inhibitors suggests high efficacy and a rapid and potent effect on itch. The JAK inhibitors are also attractive for their administration schedule and route—once daily by mouth—and because they are rapidly cleared from the body. Adverse events (AEs) may be the limiting factor for their use. The most common AEs in clinical trials have been nausea, headache, and acne.
“The opportunity to easily start and stop the JAK inhibitors suggests they may have a role for controlling AD flares or seasonal involvement,” Paller said.
“It remains to be seen if there are differences in side effect profiles among the JAK inhibitors that will favor using any one of these agents in the pediatric population. The good news is that there are no signals for malignancy, thromboembolism, or high-risk infections with their administration in the AD trials, although it remains to be seen what risks might emerge post-approval with expanded and longer-term use,” Paller said.
Disclosure:
Paller is an investigator and/or consultant for companies that market and/or are developing systemic treatments for AD.







