- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Platelet-Rich Plasma in Hair Transplantation Shows Success in Patients with Androgenetic Alopecia
Author(s):
Key Takeaways
- PRP enhances the microenvironment for hair growth by promoting cell growth, inhibiting apoptosis, and reactivating dormant follicles.
- The study showed higher follicle survival and growth rates in the PRP group compared to the control group.
Patients achieved excellent new hair growth and strength when combining PRP with drug therapy after hair transplantation surgery.
Image Credit: © Ihor - stock.adobe.com
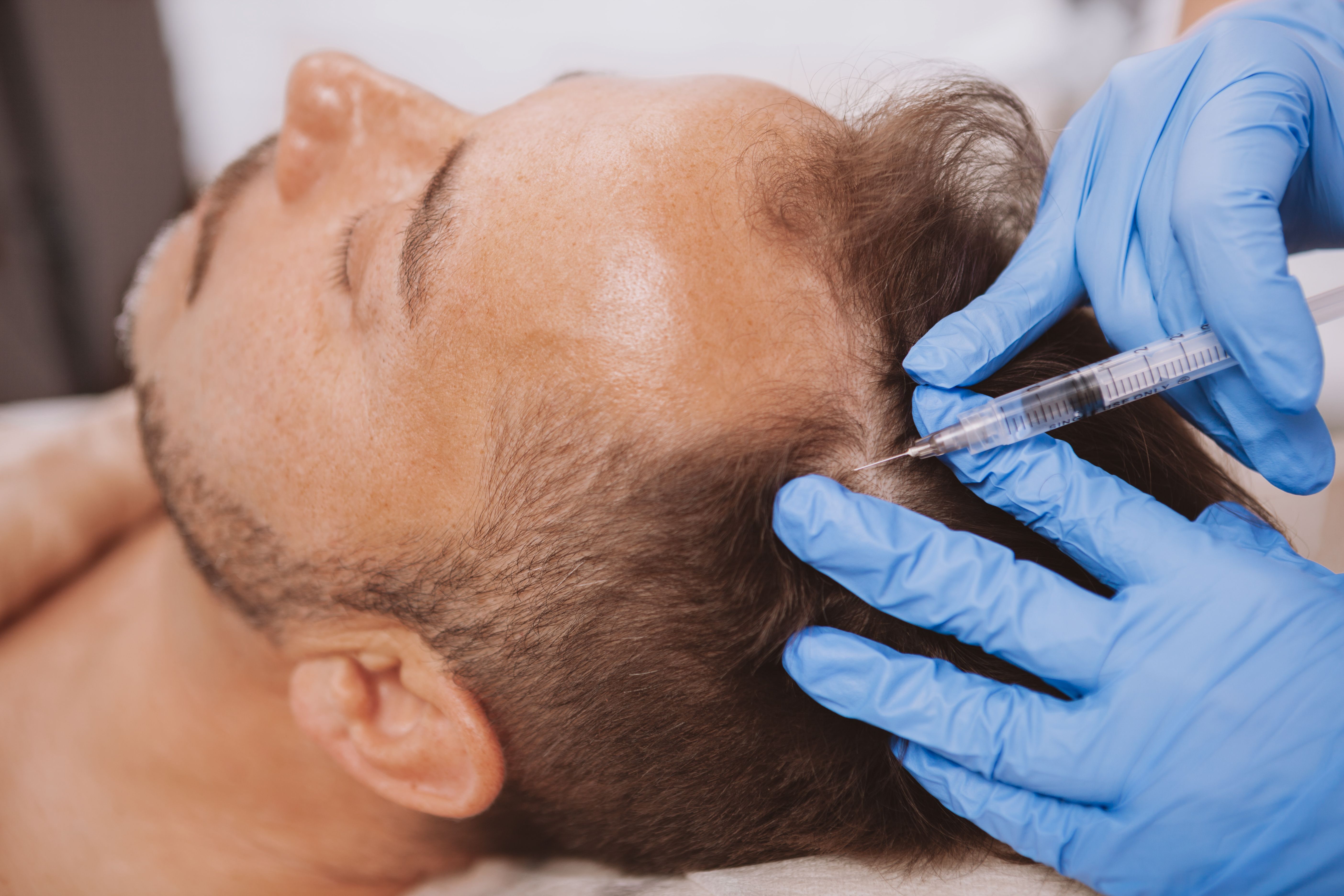
Researchers evaluated the combination of platelet-rich plasma (PRP) with minoxidil and finasteride/spironolactone in patients with androgenetic alopecia (AGA) after hair transplantation.1 Research on the potential of PRP stimulating hair growth has been ongoing since 2006 and it has been widely applied in clinical settings.2 Currently, finasteride (for men only) and minoxidil are the only FDA-approved treatments for AGA.
“PRP improves the microenvironment of the transplanted area by promoting cell growth and differentiation, inhibiting apoptosis, and enhancing the formation of new blood vessels, making the transplant area more conducive to new hair growth,” the authors wrote.“It also provides favorable conditions for dormant follicles, reactivating them and promoting new hair growth within 3 months.”
The prospective, comparative clinical study took place between August 2019 and December 2022 and included 30 patients between the ages of 20 and 50 years. The participants were split evenly into 2 cohorts. The experimental group of 11 males and 4 males received drug therapy and PRP injections along with the hair transplantation. The control group of 13 males and 2 females only received the drug therapy in combination with the procedure.
Drug therapy was defined as the administration of oral spironolactone or finasteride combined with topical minoxidil treatment. Minoxidil was applied once a day for the first week before being upped to twice a day. Oral finasteride (1 mg) was administered once a day in male participants while female patients took 20 mg of spironolactone twice a day.
The follicle unit extraction technique was used for hair transplantation. This widely used technology is known for its minimal trauma, fast healing, and absence of linear scarring. The experimental group received approximately 15 mL of PRP before follicle extraction. With injections at 1- and 2-months post-surgery, the final PRP concentration was about 5 times the patient's own platelet count.
Preoperative hair loss was assessed via the Norwood–Hamilton grade for male patients and the Ludwig grade for female patients. The median grade for males in both groups was 4. In females, the experimental group had a median of 1 while the control group had a median of 2. There were no significant differences in baseline characteristics between the two groups.
Prior to surgery, there were no differences in results between the 2 groups. Additionally, there was no significant difference in the average number of transplanted follicular units. After the surgery, however, the experimental group achieved excellent treatment outcomes with significantly higher follicle survival and growth rates as well as improved hair strength.
At 3 months post-surgery, the follicle survival rates were 88.42% for the experimental group and 78.24% for the control group (p = 0.001). These numbers changed at 6 months post-surgery to 82.17% and 74.01% for the experimental and control groups, respectively (p = 0.002). The average time to initial hair growth was also shorter in the experimental group at 17.67 days versus the control group’s 20.07 days.
Researchers used traction tests at the 3-month mark to test the strength of the hair. In the experimental group, 13 patients were negative and 2 were positive. In the control group, 8 patients were negative and 7 were positive (p = 0.046).
Postoperative complications were found in both groups including bruising, pain, and hair curling. In the experimental group, a patient experienced transient itching at the surgical site while a patient in the control group developed folliculitis.
Although these results are promising, there is no consensus among researchers regarding the optimal platelet concentration and composition in PRP and the standardization of protocols (open versus closed methods). Because of this uncertainty, further research is recommended to determine PRP’s specific therapeutic effects using large-scale, multicenter, prospective randomized studies.
References
1. Xue P, Guo L, Dang E, et al. A Prospective and Comparative Study to Explore the Effects of Platelet-Rich Plasma in Hair Transplantation for Patients With Androgenetic Alopecia. J Cosmet Dermatol. Published online November 18, 2024. doi:10.1111/jocd.16665
2. Uebel CO, da Silva JB, Cantarelli D, Martins P. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg. 2006;118(6):1458-1466. doi:10.1097/01.prs.0000239560.29172.33






