- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Phase 2 Data of Personalized Cancer Vaccine With Adjuvant Pembrolizumab for Resected High-Risk Melanoma
Author(s):
Data from the KEYNOTE-942 trial was presented at the American Association for Cancer Research Annual Meeting 2023.
Photo courtesy of the CDC Public Health Image Library
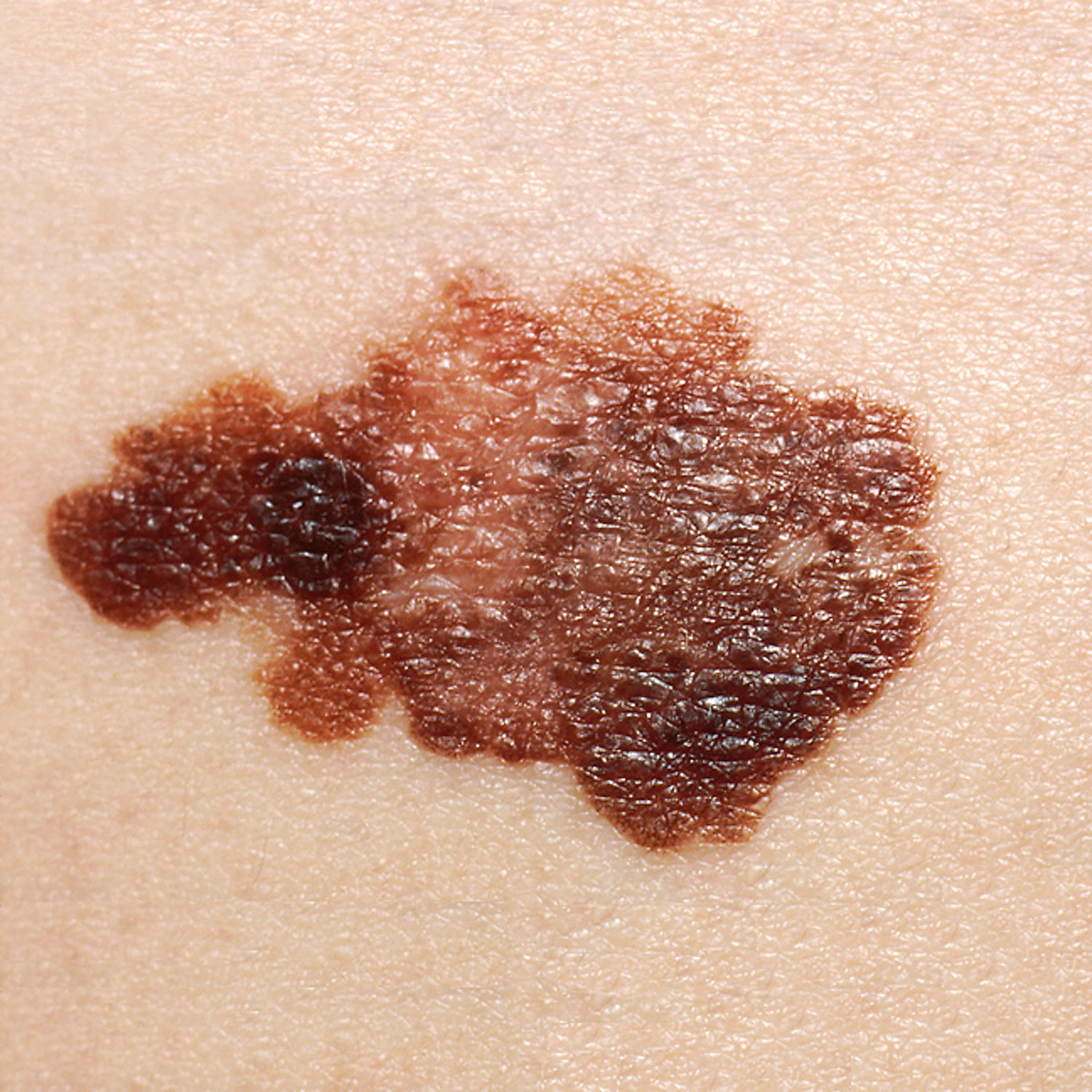
Earlier this week, the American Association for Cancer Research (AACR) Annual Meeting 2023 kicked off in Orlando, Florida. The 6-day conference covers a wide variety of cancer sciences and medicine topics across multiple session tracks. Today, new phase 2 safety and efficacy data was presented at AACR 2023 on mRNA-4157 (V940; Merck and Moderna), a personalized cancer vaccine combined with pembrolizumab (Merck), an anti-PD-1 therapy, as adjuvant treatment for high-risk melanoma.1
The presentation abstract noted that targeting of mutation-derived epitopes, or neoantigens, by T cells has been shown to drive anti-tumor immune responses. MRNA-4156 encodes up to 34 patient-specific tumor neoantigens. Merck and Moderna have partnered together to determine if mRNA-4157 can be paired with adjuvant pembrolizumab to improve recurrence free survival in patients with resected stage IIIB/IIIC/IIID/ and IV melanoma.
During the randomized, open-label, phase 2 KEYNOTE-942 (NCT03897881) clinical trial, 107 patients received mRNA-4157 combined with pembrolizumab and 50 patients received pembrolizumab monotherapy. Recurrence or death was reported in 24 out of 107 patients (22.4%) in the combination arm and in 20 out of 50 patients (40%) in the monotherapy arm, with a median follow-up of 101 and 105 weeks. The 18-month recurrence free survival rates (95% CI) were 78.6% (69.0% at 101 weeks and 85.6% at 105 weeks) in the combination arm and 62.2% (46.9% at 101 weeks and 74.3% at 105 weeks) in the monotherapy arm.
The combination arm showed protocol defined statistical significance and a clinically meaningful improvement in recurrence free survival compared to pembrolizumab monotherapy. Additionally, the combination arm had a reduction in the risk of recurrence or death by 44%. Most reported treatment-related adverse events were grade 1/2, with the number of patient-reported treatment-related grade ≥3 adverse events being similar between the arms (25% vs 18%, respectively). Fatigue was the most common mRNA-4157-related grade 3 adverse event, and no mRNA-4157-related grade 4 or 5 adverse events were reported.
Eligible patients with completely resected, high-risk cutaneous melanoma were randomly assigned 2:1 to receive mRNA-4157 plus pembrolizumab or pembrolizumab as monotherapy. One milligram of mRNA-4157 was administered intramuscularly every 3 weeks for a total of 9 doses, and 200 mg of pembrolizumab was injected intravenously every 3 weeks for up to 18 cycles. The primary endpoint of KEYNOTE-942 was recurrence free survival in the overall intent-to-treat population. Safety was evaluated as a key secondary endpoint.
The study investigators concluded that “mRNA-4157 in combination with pembrolizumab as adjuvant therapy for resected high-risk melanoma significantly prolonged [recurrence free survival] compared to pembrolizumab without an increase in clinically meaningful adverse events. These results are the first to demonstrate improvement of [recurrence free survival] over adjuvant standard of care PD-1 blockade in resected high-risk melanoma and provide the first randomized evidence that a personalized neoantigen approach is potentially beneficial for cancer patients.”
Reference
- Weber J. A personalized cancer vaccine, mRNA-4157, combined with pembrolizumab versus pembrolizumab in patients with resected high-risk melanoma: Efficacy and safety results from the randomized, open-label phase 2 mRNA-4157-P201/Keynote-942 trial. Presented at the American Association for Cancer Research Annual Meeting 2023; April 14-19, 2023; Orlando, FL.





