- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Nonthermal energy shows promise for back acne
Author(s):
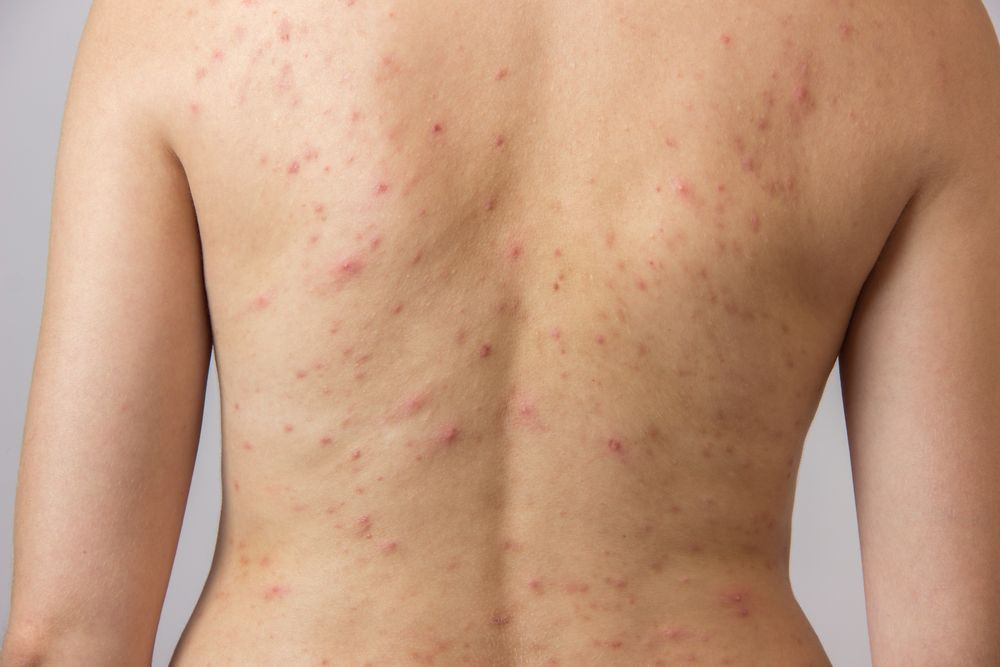
Early results from the first patients treated for back acne with Nano-Pulse Stimulation in a controlled study show that use of this nonthermal energy modality is providing rapid and sustained clearance of inflammatory lesions, reported Mark Nestor, M.D., Ph.D., at the 2019 annual meeting of the American Society for Dermatologic Surgery.
“NPS causes selective redution of sebaceous gland cells, which explains the rationale and efficacy that is being observed," says Dr. Nestor. (©OksanaVolina/Shutterstock.com)
Dr. Nestor

Early results from the first patients treated for back acne with Nano-Pulse Stimulation (“NPS”, Pulse Biosciences) in a controlled study show that use of this nonthermal energy modality is providing rapid and sustained clearance of inflammatory lesions, reported Mark Nestor, M.D., Ph.D., at the 2019 annual meeting of the American Society for Dermatologic Surgery.
RELATED: Risk of scarring in adults with persistent acne
“NPS causes selective reduction of sebaceous gland cells which explains the rationale and the efficacy that is being observed with its use for treatment of acne,” says Dr. Nestor, director for Cosmetic Enhancement and Center for Clinical and Cosmetic Research, Aventura, Fla., and voluntary professor, department of dermatology and cutaneous surgery, University of Miami Miller School of Medicine, Miami. “The study is ongoing. More patients are being enrolled and we are working to optimize the energy level and the technology, as well as exploring the use of new tips that will enable treatment of a larger surface area.”
NPS is performed with a proprietary device (CellFX System, Pulse Biosciences) that uses a sterile treatment tip containing microneedles to deliver a series of timed, ultrashort (nanosecond), high voltage, electrical energy pulses to the target site. The nonthermal energy causes disruption of cellular membranes and internal cell organelles, leading to the activation of pathways that result in regulated cell death. Histological studies have shown that NPS treatment selectively affects cellular structures within the treatment zone, including melanocytes, sebaceous glands and hair follicles, without affecting acellular structures, such as elastic fibers and collagen, or adjacent tissue.
“The melanocytes, however, reappear over time and reach levels comparable to those seen in untreated controls by one month after NPS treatment,” Dr. Nestor says.
READ MORE: Energy-based options for acne
“In large clinical studies, this technology has demonstrated 90% to 99% efficacy in clearing sebaceous hyperplasia. These incredible results spurred our interest in investigating its use for treating acne,” Dr. Nestor says. “We chose to study acne vulgaris on the back because it is typically challenging to treat and because the back provides a relatively large surface area that allows for an intrapatient control design that compares changes after NPS treatment vs. sham and untreated control sites."
The study of NPS for back acne is being conducted by Dr. Nestor and Bruce Katz, M.D., clinical professor of dermatology, Icahn School of Medicine at Mount Sinai, and director, Juva Skin & Laser Center, New York. It is comparing the effects of NPS against sham treatment and an untreated control site. It has a planned enrollment of up to 20 patients and follow-up of 90 days posttreatment.
RELATED: Topical probiotics may restore skin microflora, decrease acne lesions
To be eligible for participation, patients must have a minimum of three areas measuring 7x 7 cm of active acne on the back, each containing at least five inflammatory lesions rated moderate to severe. The lesion count must also be similar across the three areas on the back.
NPS energy titration is performed at the baseline visit. One week later, treatment with NPS or sham is applied to one-half of the designated area, and the remaining section is treated on day 21. Patients return for safety and efficacy evaluations at 30, 60 and 90 days thereafter.
Data are available from six patients who each had at least seven to 10 inflammatory lesions within each of the three target sites on the back. The applied energy dose was already titrated down in this first cohort of patients, but across the dose range studied, benefit was noted by 30 days and has been sustained with follow-up reaching up to eight months.
“Our initial data show an 89% lesion reduction in the treated areas,” Dr. Nestor says. “The durability of the responses is expected based on the theory that NPS would be an effective treatment for acne because it destroys the sebaceous gland."
READ MORE: Adult, teen acne differ
Post-inflammatory hyperpigmentation was the only significant side effect observed so far. The hyperpigmentation resolved over time, was greater in patients with a darker skin type (patients with up to Fitzpatrick skin type 4 are eligible for enrollment), and has been minimized as the energy dose was reduced. Â






