- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
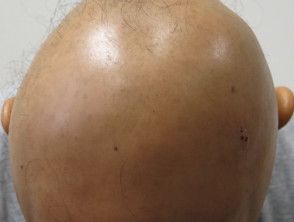
Nektar Announces Initiation of Phase 2b Study for Rezpegaldesleukin in Severe, Very Severe Alopecia
Author(s):
The study will evaluate rezpegaldesleukin and its effect on Severity of Alopecia Tool scoring by the conclusion of a 36-week period.
Nektar Therapeutics announced today it has commenced its phase 2b clinical trial to assess the potential of rezpegaldesleukin (REZPEG) in treating patients with severe to very severe alopecia areata.1
The novel therapeutic approach aims to mitigate the inflammatory response while restoring immune balance by bolstering functional T regulatory cells and engaging multiple immunoregulatory pathways.
The phase 2b trial, spanning 36 weeks, will enroll 84 participants with severe to very severe alopecia areata in a global, randomized, double-blind, placebo-controlled design.
Investigating 2 distinct dosing regimens of REZPEG against placebo, the study will evaluate both efficacy and safety parameters, with a focus on the mean percent improvement in the Severity of Alopecia Tool (SALT) as the primary endpoint at week 36.
Secondary endpoints include assessing the proportion of participants achieving a 50% or greater reduction in SALT at various time points throughout the study.
"The start of this phase 2b study is another significant milestone for Nektar as we advance REZPEG, a potentially transformative new mechanism for alopecia areata and other auto-immune disorders," said Mary Tagliaferri, MD, chief medical officer at Nektar Therapeutics, in a news release.1
"Current treatments available have high relapse rates and carry potential safety challenges. As a result, there is a high unmet need for durable and well-tolerated treatment options that target the underlying dysfunction of the immune system in these patients," Tagliaferri said. "We believe there's an opportunity for REZPEG to emerge as a novel biologic mechanism for alopecia patients and we look forward to our topline data from this study."
Topline data from the study in alopecia is anticipated in the first half of 2025.
Currently, REZPEG is also in phase 2b development for atopic dermatitis (AD), (NCT06136741).2
In September, Nektar announced promising data stemming from its phase 1b trial of REZPEG in patients with moderate-to-severe AD.3
Over a 12-week period, patients treated with 2 different doses of rezpegaldesleukin experienced significant improvements in their eczema symptoms compared to those on placebo. Both doses led to considerable reductions in Eczema Area and Severity Index (EASI) scores, with the higher dose showing greater improvement.3
References
- Nektar Therapeutics announces initiation of phase 2b clinical study evaluating rezpegaldesleukin in patients with severe to very severe alopecia areata. News release. PR Newswire. March 5, 2024. Accessed March 5, 2024. https://www.prnewswire.com/news-releases/nektar-therapeutics-announces-initiation-of-phase-2b-clinical-study-evaluating-rezpegaldesleukin-in-patients-with-severe-to-very-severe-alopecia-areata-302079957.html
- ClinicalTrials.Gov. A phase 2b study to evaluate rezpegaldesleukin (REZPEG) in the treatment of adult patients with moderate-to-severe atopic dermatitis (REZOLVE-AD). Accessed March 5, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT06136741
- Nektar Therapeutics announces promising new data from phase 1b study of rezpegaldesleukin in moderate-to-severe atopic dermatitis. News release. PR Newswire. September 13, 2023. Accessed March 5, 2024. https://www.prnewswire.com/news-releases/nektar-therapeutics-announces-promising-new-data-from-phase-1b-study-of-rezpegaldesleukin-in-moderate-to-severe-atopic-dermatitis-301925787.html.