- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
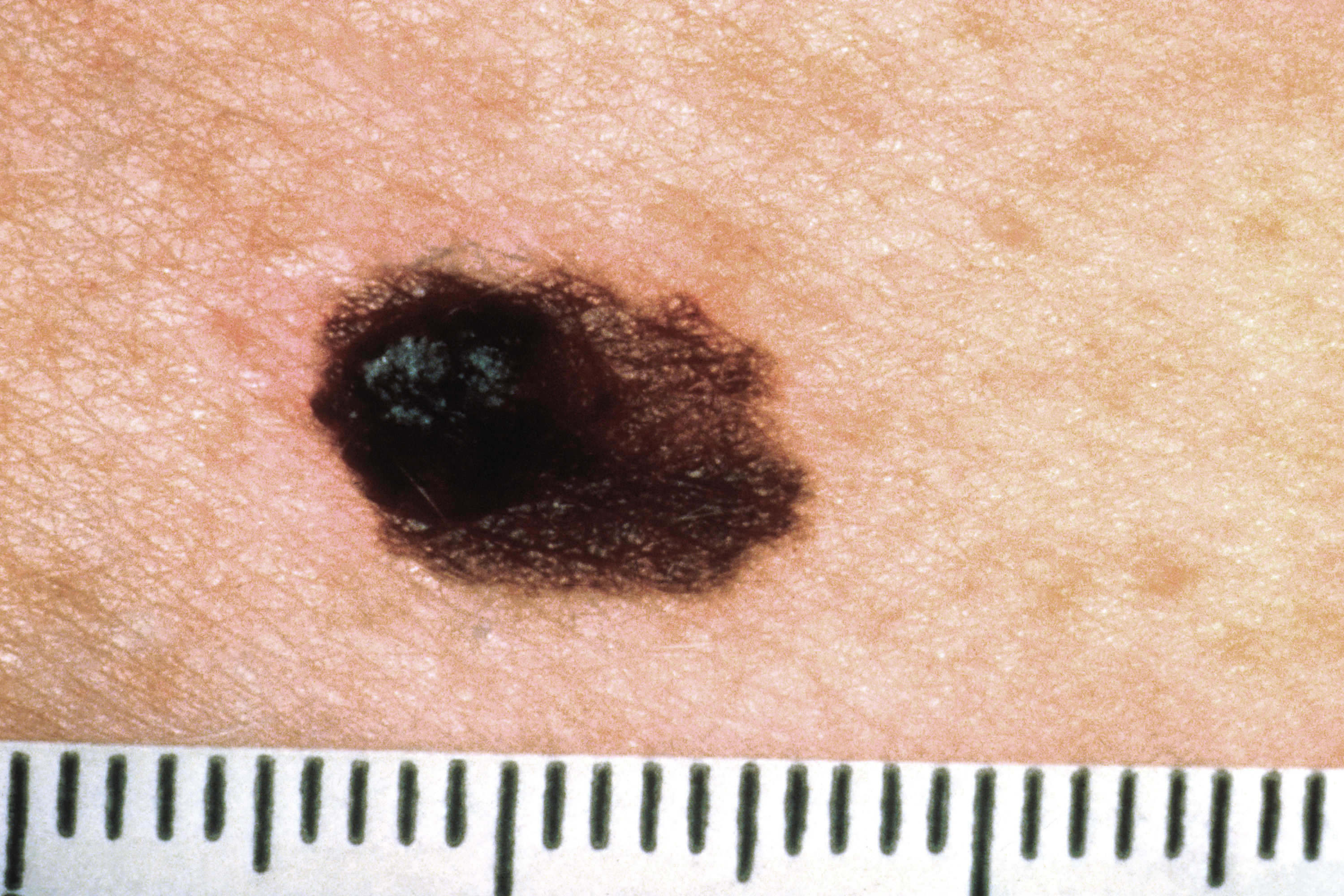
Merck and Moderna Initiate Phase 3 Study Evaluating V940 in Combination With Pembrolizumab for Resected Stage IIB-IV Melanoma
Author(s):
V940-001 is the first phase 3 study of a planned comprehensive clinical development program initiated after the positive primary analysis of the phase 2b KEYNOTE-942/mRNA-4157-P201 trial.
Merck and Moderna recently announced the initiation of their pivotal phase 3 V940-001 clinical trial evaluating V940 (mRNA-4157; Moderna), an investigational individualized neoantigen therapy, in combination with pembrolizumab (Keytruda; Merck), an anti-PD-1 therapy, as an adjuvant treatment for patients with resected high risk (stage IIB-IV) melanoma.1 The first patients are now enrolling in Australia as global recruitment for V940-001 has officially started.
V940-001 (NCT05933577), a global, randomized, double-blind, placebo- and active-comparator-controlled study, is designed to evaluate the safety and efficacy of V940 in combination with pembrolizumab compared to pembrolizumab alone. Approximately 1089 patients at more than 165 sites and in over 25 countries are expected to be enrolled in the trial.
The primary endpoint of V940-001 is recurrence-free survival (RFS), and secondary endpoints include distant metastasis-free survival (DMFS), overall survival, and safety. Following complete surgical resection, patients aged 18 years and older will be randomized 2:1 to receive V940 1mg every 3 weeks for up to 9 doses, and pembrolizumab 400mg every 6 weeks for up to 9 cycles, versus pembrolizumab alone for approximately one year until disease recurrence or unacceptable toxicity, or for a total treatment duration of up to approximately 56 weeks, whichever occurs first. The primary endpoint is RFS, defined as the time from randomization to any type of disease recurrence as assessed by the investigator, or death due to any cause.
Eligibility criteria for V940-001 include patients who have surgically resected and histologically/pathologically confirmed diagnosis of stage IIB or IIC, III or IV cutaneous melanoma, patients who have not received any prior systemic therapy for their melanoma beyond surgical resection, and no more than 13 weeks have passed between final surgical resection.
Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 trial, the US Food and Drug Administration and European Medicines Agency granted Breakthrough Therapy Designation and the Priority Medicines scheme, respectively, for V940 in combination with pembrolizumab for the adjuvant treatment of patients with high-risk melanoma. Merck and Moderna presented the study’s primary endpoint, RFS, in April 2023, at the American Association for Cancer Research Annual Meeting and presented the study’s key secondary endpoint, DMFS, in June 2023, at the American Society of Clinical Oncology Annual Meeting.
Reference
- Merck and Moderna initiate phase 3 study evaluating V940 (mRNA-4157) in combination with Keytruda (pembrolizumab) for adjuvant treatment of patients with resected high-risk (stage IIB-IV) melanoma. Merck. July 26, 2023. Accessed July 27, 2023. https://www.merck.com/news/merck-and-moderna-initiate-phase-3-study-evaluating-v940-mrna-4157-in-combination-with-keytruda-pembrolizumab-for-adjuvant-treatment-of-patients-with-resected-high-riskstage-iib-iv-melanom/