- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
IL-17 potential therapeutic target for pustular psoriasis
Author(s):
Researchers performed a systematic review of available reports on the therapeutic benefit of interleukin (IL)-17/T-helper 17 (Th17) axis inhibitors in patients with generalized pustular psoriasis (GPP). Read what they discovered in this article.
(©JosephslTanMatt/Shutterstock.com)
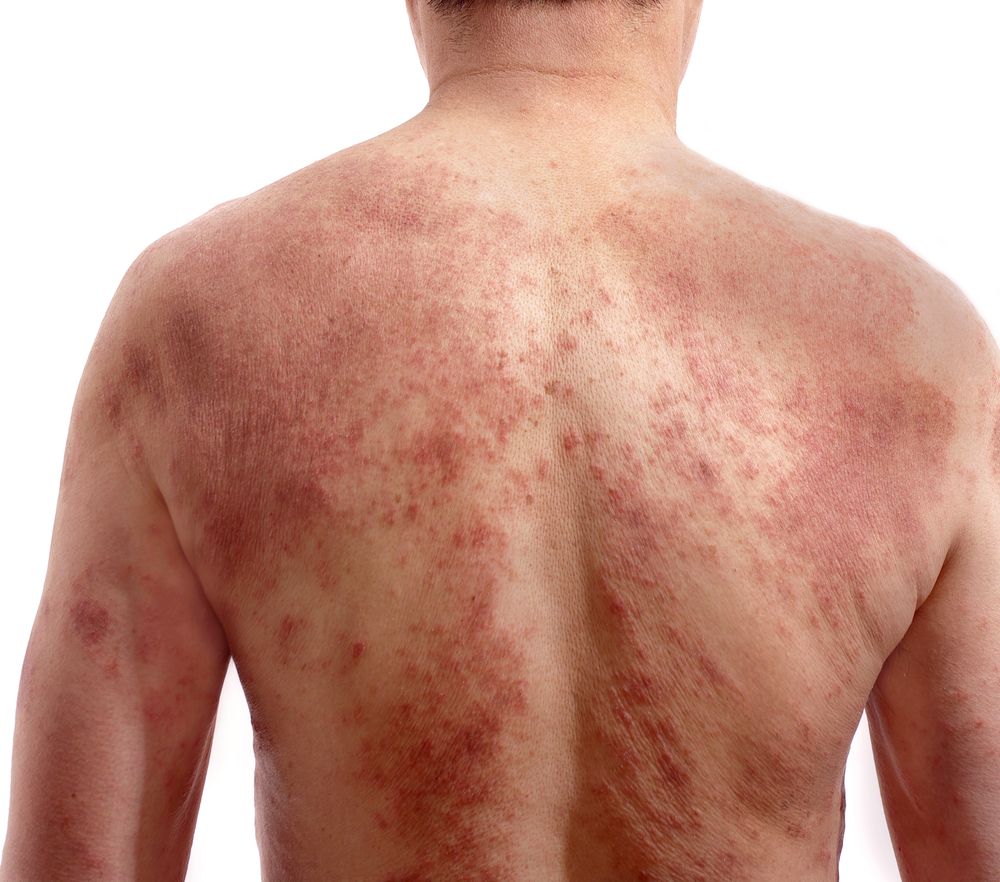
Findings from published reports demonstrate therapeutic benefit of interleukin (IL)-17/T-helper 17 (Th17) axis inhibitors in patients with generalized pustular psoriasis (GPP). Establishing a role of these biologic agents in the management of GPP, however, will require additional research assessing their efficacy and safety, according to Plachouri et al. who undertook a systematic review of the available evidence on this topic.
NEW: Socioeconomic factors affect psoriasis treatment outcomes
In their report, the investigators stated that other conventional systemic therapies (i.e., retinoids, cyclosporine, and methotrexate) and other biologics (i.e., tumor necrosis factor α inhibitors and IL-12/23 inhibitors) have been associated with variable results when used to treat GPP. They noted that the pathogenesis of GPP is not fully understood, but that mutations in the IL36RN gene that encodes the IL-36 receptor antagonist protein and in CARD14 (caspase recruitment domain-containing protein) are thought to be associated.
Interest in IL-17 inhibition relates to reports that serum and mRNA levels of IL-17 are significantly higher in patients with pustular psoriasis than in patients with nonpustular disease. Plachouri et al. pointed out that because IL-17 is a potent neutrophilic chemotactic factor, the elevated levels of serum and mRNA levels of IL-17 in patients with pustular psoriasis is consistent with the increased neutrophilic infiltration seen in this particular psoriatic subtype.
What’s in the literature?
To examine the evidence for IL-17 blockade as a treatment for GPP, the authors performed a review following recommendations of the Preferred Reporting Items for Systemic Reviews. They searched the Embase, MEDLINE (PubMed), and Scopus databases for English language articles published between 2012 and 2019 to identify papers relating to GPP and the use of secukinumab, ixekizumab and brodalumab. The search identified 88 articles; 38 fit the inclusion criteria for review, and the authors summarized their findings.
NEW: Considerations for psoriasis patients with skin of color
Secukinumab was evaluated in a Japanese open-label phase III study enrolling 12 adult patients, of which only 3 received secukinumab as monotherapy. Treatment with secukinumab was associated with early benefit, significant improvement at the week 16 endpoint, durable responses through week 52, and no unexpected adverse events.
Other articles describing secukinumab included one case series comprised of six patients, eight reports of single adult cases, and four case reports describing pediatric patients. Overall, these reports showed rapid remission of GPP with the use of secukinumab, and authors of the case series noted that it could be an effective therapeutic option independent of IL36RN mutation status.
RELATED: Phototherapy safe, effective for psoriasis
Ixekizumab was evaluated in two Japanse open-label, phase III trials, each including five adult patients. Follow-up duration was 24 weeks in one trial and extended to over 3 years in the other study. In both cohorts, ixekizumab was well-tolerated and associated with early benefit that was sustained in most patients. Three other published reports were identified that also described rapid improvement of GPP in single patients treated with ixekizumab.
Only a single paper was identified that described brodalumab treatment for patients with GPP. The article reported on a Japanese open-label, phase III study that included 12 adults. Significant improvement or remission was obtained in 83.3% of patients at week 12 and in 91.7% of patients at week 52.
RELATED: Interleukin-29 for inflammatory autoimmune diseases
Plachouri et al. also identified a single case report of a patient deemed to have possible secukinumab-induced paradoxical pustular psoriasis. Two papers were found that described a total of 22 patients who developed pustular disease after discontinuing brodalumab treatment for plaque psoriasis. Authors of one paper postulated that abrupt withdrawal of TH17/IL-17 axis suppression could be the underlying mechanism. They further noted that compared with secukinumab and ixekizumab, development of pustular lesions may be more likely after discontinuing brodalumab because it has a broader spectrum of action for suppressing the TH17/IL-17 pathway. They explained that whereas secukinumab and ixekizumab target the IL-17A ligand, brodalumab targets the IL-17 receptor A and blocks the action of multiple members of the IL-17 family, including IL-17A, F, E, C, and A/F.
References:
Plachouri KM, Chourdakis V, Georgiou S. The role of IL-17 and IL-17 receptor inhibitors in the management of generalized pustular psoriasis. Drugs Today. 2019;55(9):587-593.






