- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
GLA Offers Promise as Add-on Therapy to Minocycline
Author(s):
Patients with rosacea can get better results by supplementing standard minocycline oral therapy with γ-linolenic acid (GLA), according to findings from a recent study.
Patients with rosacea can get better results by supplementing standard minocycline oral therapy with γ-linolenic acid (GLA), according to findings from a recent study.1 This add-on therapeutic also may ameliorate adverse effects (AEs) such as nausea, gastrointestinal concerns, and skin staining associated with some rosacea therapies, potentially improving adherence over long- term treatment, wrote the study authors.
For the study, Ji Hyun Kim, MD, of the Department of Dermatology at Ulsan University Hospital, University of Ulsan College of Medicine, in Korea, and colleagues investigated the efficacy and safety of adding GLA to minocycline compared with minocycline alone to treat rosacea by restoring the skin barrier function.
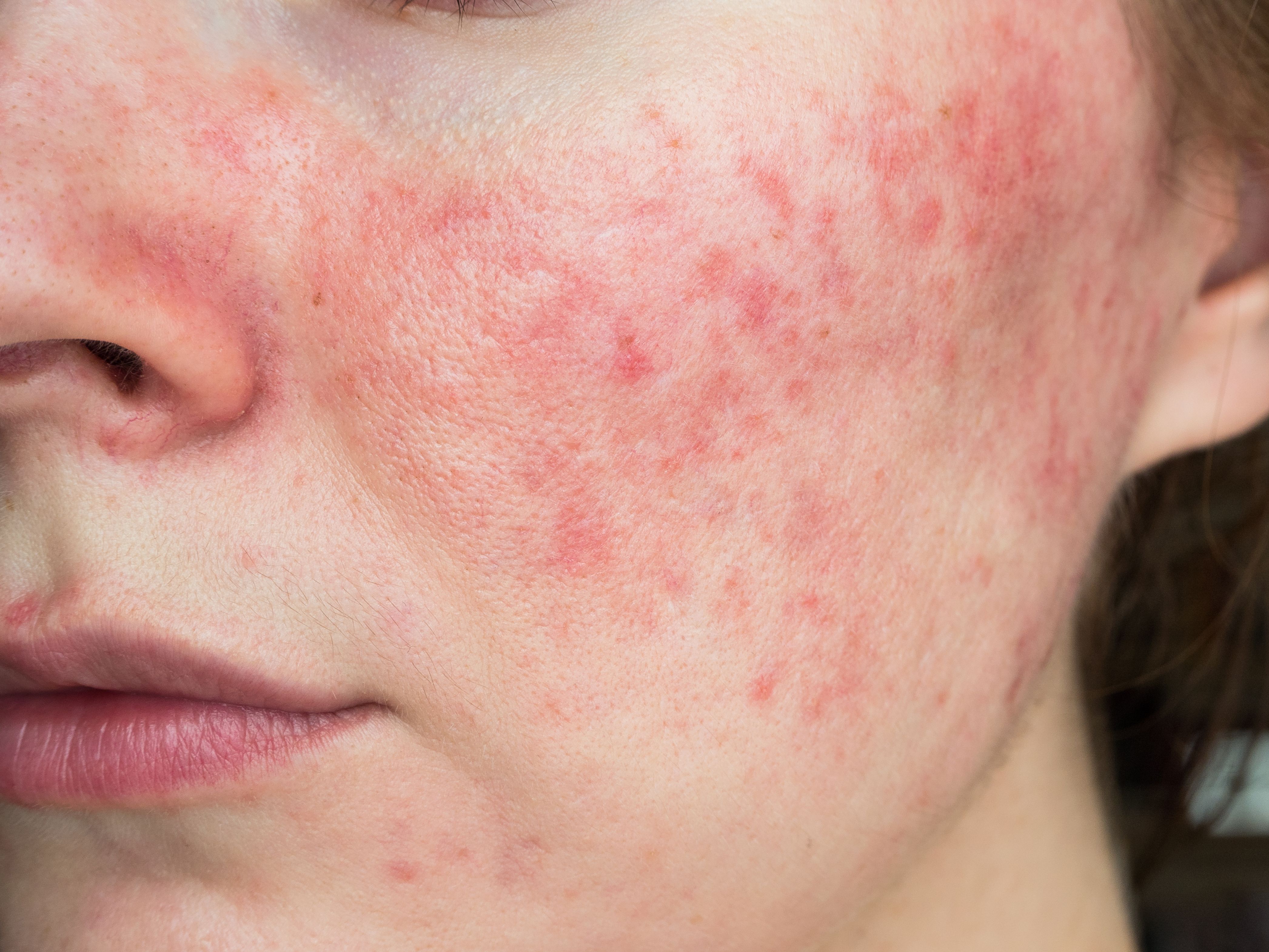
Current therapeutics generally address this inflammatory skin disease’s mosaic of cutaneous signs and symptoms including facial erythema; edema; flushing, with or without papules or pustules; and telangiectasias. These symptoms typically occur around the nose, cheeks, forehead, and chin. Skin barrier dysfunction also can result in further irritation and exacerbate symptoms.
“Recent studies2 have highlighted the skin barrier abnormalities in rosacea, which induce chronic perivascular, dermal, and epidermal inflammation,” the authors wrote. “This chronic inflammation leads to increased transepidermal water loss (TEWL) and decreased stratum corneum hydration, thus initiating a vicious cycle.”
The result: a functionally impaired stratum corneum permeability barrier, which can lead to dryness, stinging, burning, and skin irritation. Known for its anti-inflammatory effect, GLA, an essential omega-6 fatty acid, has been shown to regenerate a defective skin barrier, according to the authors. GLA can be used both topically and systemically.
The prospective, double-blind, placebo-controlled trial included 31 adults with rosacea who were randomized to receive 320 mg/day GLA (Evoprim; Biochemix; n = 16) or placebo (n = 15) in addition to 100 mg/day of minocycline for 8 weeks. The study included both erythematotelangiectatic and papulopustular rosacea subtypes. The investigators assessed clinical severity and improvements from treatment at weeks 0, 4, 8, and 12 using investigator global assessment (IGA) and patient global assessment (PGA) scores. They also measured and assessed changes post treatment of several biophysical parameters, including melanin index, erythema index, TEWL, lipid concentration, and stratum corneum hydration.
Results showed that a higher proportion of patients who received GLA compared with the placebo group achieved treatment success (IGA ≤ 1) at week 8 (68.75 % vs 33.33 %) and patient satisfaction (PGA ≥ 3) at weeks 8 (75.0 % vs 40.0 %) and 12 (81.3 % vs 46.6 %). Data revealed a trend toward improvement in erythema index, melanin index, TEWL, and stratum corneum hydration in both groups over the 12-week study period. Results also demonstrated a significant difference in TEWL and stratum corneum hydration over time between the 2 groups. The investigators found GLA to be generally well tolerated, and no serious AEs were recorded in either treatment group.
“GLA supplementation as an add-on to minocycline has the additional benefit of improving TEWL and stratum corneum hydration by restoring skin barrier function, which results in
[higher] patient satisfaction,” the authors wrote. According to the investigators, the results demonstrate statistically significant and clinically meaningful improvements with GLA in rosacea symptoms of both erythematotelangiectatic and papulopustular rosacea subtypes, as well as skin barrier restoration. They suggest that future studies include larger patient cohorts and subgroup analysis between rosacea subtypes, which could help reveal differences in the additive effect of GLA among subtypes.
Disclosures:
The authors report no relevant disclosures.
References:
1. Kim JH, Oh YW, Kim DH, Seo BH, Suh HS, Choi YS. A randomized, placebo-controlled trial of gamma linolenic acid as an add-on therapy to minocycline for the treatment of rosacea. Ann Dermatol. 2020;32(6):466-472. doi:10.5021/ad.2020.32.6.466
2. Addor FA. Skin barrier in rosacea. An Bras Dermatol. 2016;91(1):59-63. doi:10.1590/abd1806-4841.20163541