- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
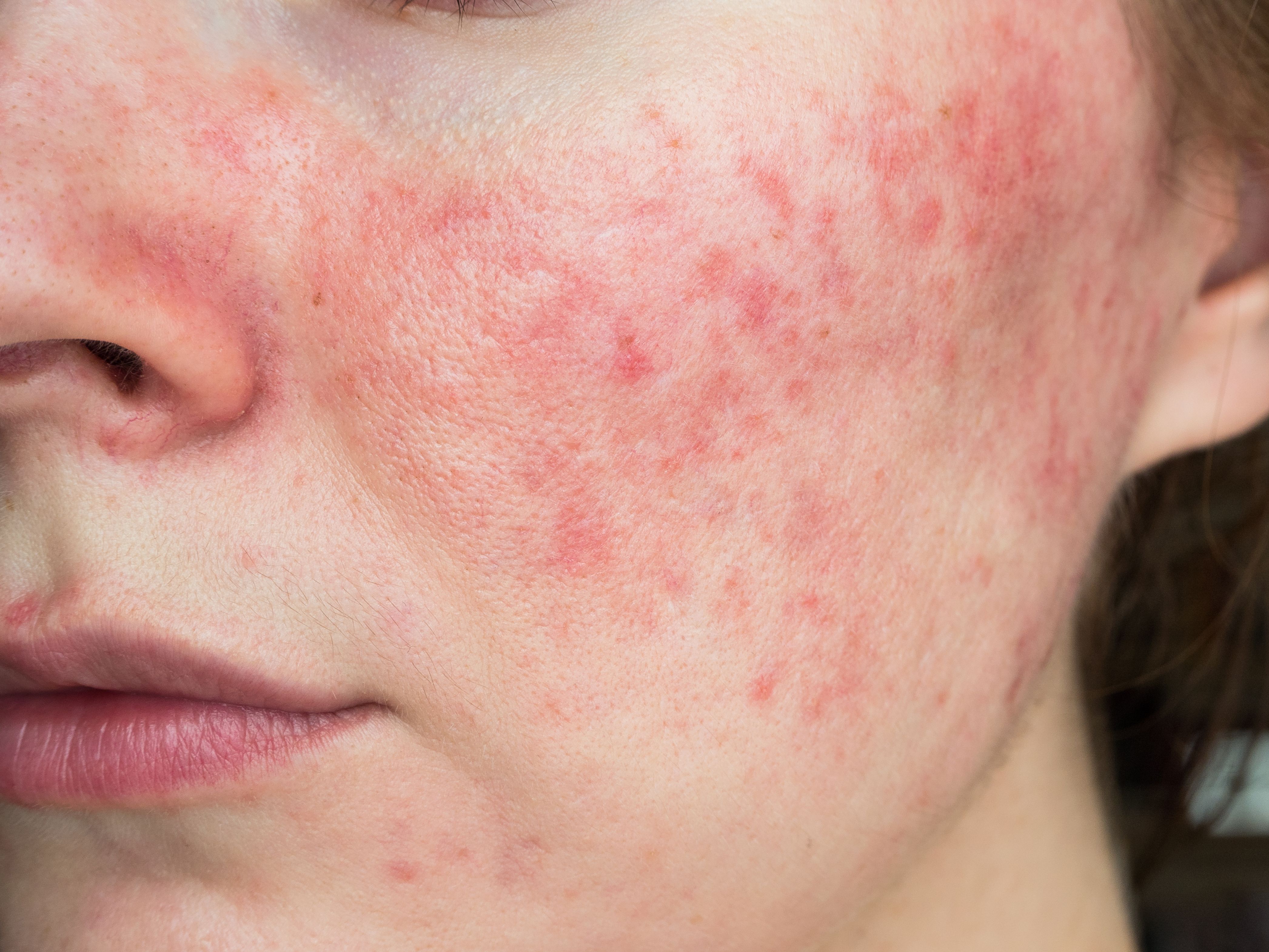
Full Pipeline, Customized Diagnoses Expand Options for Rosacea
Author(s):
Challenges with overlaps in diagnosis and classification based on rosacea’s 4 subtypes are creating a new focus on features of the condition in each patient. A pipeline of new drugs awaiting FDA approvals could further expand options.
New developments in rosacea are not limited to treatment. There is an ongoing paradigm shift in how rosacea is diagnosed and classified.
“We are getting away from the old subtypes,” said Julie Harper, MD, FAAD, owner of the Dermatology and Skin Care Center of Birmingham in Alabama. “No longer are we going to be talking about erythematotelangiectatic vs papulopustular rosacea vs phymatous rosacea. We now are simply subtyping lesions.”
Julie Harper, MD, FAAD

Often, when examining a patient, “all of those subtypes overlap,” said Harper, who spoke on what’s new in rosacea at the 2021 American Academy of Dermatology (AAD) Summer Meeting. “It is imperative that we document and observe all of the features of rosacea so we can treat the patient holistically.”1
Harper noted that the pathogenesis of rosacea encompasses the pathway of neurogenic inflammation, which likely does not travel through the innate immunity channel but rather through transient receptor potential channels. “The end result is vasodilation,” Harper told Dermatology Times®. “If a crucial component of rosacea is vasodilation, then it would stand to reason that an important part of treatment is vasoconstriction.”
Zilxi (VYNE Therapeutics) is minocycline topical foam, 1.5%, that received FDA approval last year. The minocycline itself is lipophilic, and thus in an oil base. “There is good clinical trial data that show the foam decreases inflammatory lesion count and is well tolerated,” Harper said. Clinical trials showed that at week 12, patients achieved more than a 60% reduction in inflammatory lesions with Zilxi, and roughly 50% of patients were clear or almost clear after 12 weeks of monotherapy.2,3
“When we think of minocycline, we think of it as being an antibiotic,” Harper said. “But in rosacea, we do not have a known pathogen, so we are using minocycline because of its anti-inflammatory properties.”
Because Zilxi is yellow and oily, it is more practical for patients to apply the once-daily foam at bedtime. “The product actually moisturizes and does not irritate the skin, even from day 1,” Harper said. “It is a treatment that has been very well accepted.” However, a potential negative of Zilxi is that its yellow color can stain fabric, such as pillowcases. “So, you do need to warn patients,” she said.
Epsolay (Sol-Gel Technologies/Galderma) is a 5% microencapsulated benzoyl peroxide cream that is pending FDA approval. Phase 3 trials showed a good reduction in inflammatory lesions and a high success rate of clearance or near-clearance for once-daily treatment. The baseline lesion count was 26, for which there was a reduction to 6 lesions at 12 weeks in 1 of the 2 studies.4,5
The medication was also very well tolerated by patients due to the microencapsulation of the benzoyl peroxide, “which gives it a slower release on the skin over time,” Harper said. “We think of benzoyl peroxide as being irritating; however, because of the way the drug is formulated, it will not release a high concentration right off the bat.”
Why Epsolay is effective is unknown, according to Harper. “We think of benzoyl peroxide as killing bacteria, yet we do not think of bacteria with rosacea,” she said. “I wonder if this drug is going to make us revisit the pathogenesis of rosacea and perhaps rethink the role of microorganisms.”
Additionally, in the pipeline is a novel proteasome inhibitor, ACU-DI (Accuitis). “A proteasome inhibitor works basically by inhibiting NF-KB, which is a transcription factor that turns on more inflammation,” Harper said. “The mechanism of this drug allows inflammation to decrease.”
In studies to date, the topical medication was applied twice daily. A 14-week proof-of-concept study found that of 27 patients randomized to ACU-DI, 92% had reduction in inflammatory lesions and 27% achieved a 2-plus grade reduction for clear to near clear in the 5-point Investigator’s Global Assessment (IGA) scale.6 Canfield photography also showed an improvement in erythema over time.
A second rosacea medication in the pipeline is the DFD-29 program (Journey Medical Corp/Dr Reddy’s Laboratories Ltd), consisting of minocycline extended-release capsules, 40 mg. “The minocyclines are not new to rosacea,” Harper said. “But we do not have oral minocycline.”
A phase 2 study (NCT03340961) compared DFD-29 to Oracea (Galderma), a 40-mg doxycycline modified release capsule, and placebo. “Although there were only about 50 patients in each of the arms, DFD-29 had statistically significantly better results for lesion count reduction and overall success rate vs either Oracea or placebo,” Harper said.7
Harper noted that larger studies are needed to confirm findings. “But it is not surprising that DFD-29 works as a daily medication,” she said. “However, low dose is not the same thing as being subantimicrobial. Two of the benefits of Oracea are that it is effective and does not induce antibiotic resistance. I would like to see more information about antibiotic resistance with DFD-29.” 2022 should bring progress in expanding the armamentarium that will offer even more customized solutions for treating how rosacea presents in each patient’s case.
Disclosures:
Harper is a consultant and speaker for Galderma, VYNE Therapeutics, Journey Medical, and EPI Health.
References:
1. Harper J. What’s new in rosacea. Presented at: 2021 American Academy of Dermatologists (AAD) Summer Meeting; August 5-8, 2021; Tampa, FL. Accessed August 5, 2021.
2. Gold LS, Del Rosso JQ, Kircik L, et al. Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: results of 2 phase 3, randomized, clinical trials. J Am Acad Dermatol. 2020;82(5):1166-1173. doi:10.1016/j.jaad.2020.01.043
3. Stein Gold L, Del Rosso JQ, Kircik L, et al. Open-label extension study evaluating long-term safety and efficacy of FMX103 1.5% minocycline topical foam for the treatment of moderate-to-severe papulopustular rosacea. J Clin Aesthet Dermatol. 2020;13(11):44-49.
4. Bhatia N, Werschler W, Baldwin H, et al. Long-term efficacy and safety of benzoyl peroxide cream, 5%, prepared with microencapsulation in papulopustular rosacea: results from an extension of two phase 3, vehicle-controlled trials. Poster presented at: Maui Derm Live In-Person Dermatology CME Conference and Maui Derm Connect Virtual Dermatology CME Conference; January 25-29, 2021; Maui, HI; Virtual.
5. Erlich M, Arie T, Koifman N, Talmon Y. Structure elucidation of silica-based core-shell microencapsulated drugs for topical applications by cryogenic scanning electron microscopy. J Colloid Interface Sci. 2020;579:778-785. doi:10.1016/j.jcis.2020.06.114
6. Jackson JM, Coulon R, Arbiser JL. Evaluation of a first-in-class proteasome inhibitor in patients with moderate to severe rosacea. J Drugs Dermatol. 2021;20(6):660-664. doi:10.36849/JDD.2021.5925.
7. Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791-802. doi:10.1016/j.jaad.2006.11.021