- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Frontline Forum Part 3: A Greater Understanding of the Acne Vulgaris Armamentarium
Author(s):
In part 3 of this Frontline Forum series, James Del Rosso, DO; Hilary Baldwin, MD; Neal Bhatia, MD; Christopher Bunick, MD, PhD; and Leon Kircik, MD, discuss the use of clascoterone cream 1%, differences in isotretinoin formulations, targeting sebum production, acne guidelines for patient care, and more.

An analysis of 2 phase3 randomized trials (CB-03-01/25; NCT02608450 and CB-03-01/26; NCT02608476) found that a greater proportion of patients with moderate to severe facial AV achieved treatment success at week 12 with clascoterone cream 1% compared with placebo (CB-03-01/25; 18.4% vs 9.0%; and CB-03-01/26; 20.3% vs 6.5%).4 Clascoterone cream 1% also appeared to be well tolerated, with the most common treatment-emergent adverse events being nasopharyngitis, headache, and oropharyngeal pain.4
The panelists noted that although it is unclear whether clascoterone decreases sebum quantity, it has been shown to decrease lipid and inflammatory cytokine production within the sebocytes.5 Although the specific effects have not been elucidated, the panelists predicted that clascoterone likely has an impact on sebum composition.
“Different lipids can be incredibly inflammatory,” said Bunick. “I suspect the [sebum] composition has a significant effect on inflammation, targeting other cells within the pilosebaceous unit, possibly even impacting gene expression. There are a...number of things that different lipids can have an effect on that drive acne pathogenesis.”
The panelists briefly discussed the use of sebum tape stripping, from which proteomics and lipidomics can be performed to identify the sebum composition. However, they concluded that more research is needed to use this technology effectively in AV research and clinical practice because current methods of quantifying patterns, release, and production of sebum are highly variable and can be influenced by room temperature and humidity.
Strategies for Incorporation Into Clinical Practice
Because it takes 8 to 12 weeks of use to observe the effects of clascoterone, the panelists noted that it should be used in combination with a quick-acting agent and for an extended period of time. Bunick said that he typically uses clascoterone with a retinoid and has high rates of satisfaction among his patients, with improvements first observed at 8 to 12 weeks and continued improvement in 3 to 6 months.
The panelists said that although they generally try to minimize the use of antibiotics, they may give a narrow-spectrum antibiotic in addition to clascoterone, a retinoid, and BP for a patient who wants a quick response for an upcoming event. The panelists also suggested using clascoterone with dapsone in patients who wish to avoid retinoids or clascoterone as a monotherapy for adult women in their thirties with a small number of inflammatory papillary lesions who wish to avoid spironolactone as well as the dryness and irritation associated with other acne therapies.
Optimal Use of Isotretinoin in Acne Vulgaris
Table. Pros and Cons of Different Isotretinoin Formulations
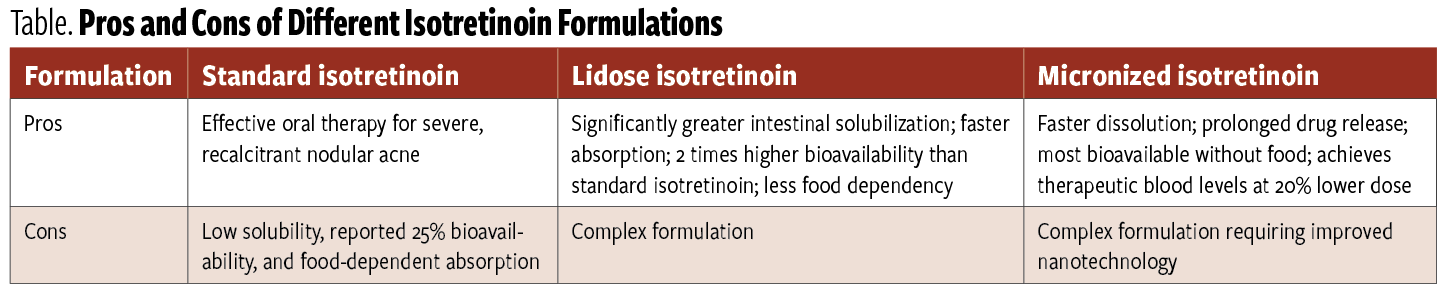
Isotretinoin is an orally administered retinoid that inhibits function and keratinization of the sebaceous gland.6 The drug is indicated for severe nodular AV that does not respond to other therapies, including systemic antibiotics.6 There are3 isotretinoin formulations and there are pros and cons to each (Table). Although a single 15- to 20-week course of treatment yields complete and durable remission in 85% of patients, the panelists noted that the public perception of isotretinoin has been tainted because its original brand-name version (Accutane) was pulled from the market by its manufacturer in 20097 (although generic versions are still available) and has been associated with severe birth defects, psychiatric disorders, pancreatitis, and Crohn disease, all of which prompted multiple lawsuits.8 Furthermore, the iPLEDGE program, which requires dispensing pharmacies and prescribers to be registered and activated within the program and for patients to register and meet all of the program’s requirements, has substantially decreased the uptake of isotretinoin since the program’s inception in 2006, said Del Rosso.6
Nevertheless, the panelists said that off-label use of isotretinoin can be valuable for patients who have been on topical and antibiotic therapy for a long duration or who are psychologically distraught by or have significant scarring from their AV. Baldwin added that the threshold for using isotretinoin should be lowered from an antibiotic stewardship perspective in particular.
The FDA-recommended dose for traditional isotretinoin is 0.5 to 1.0 mg/kg/day divided into 2 doses per day, with food (preferably a high-fat meal), for 15 to 20 weeks, although Del Rosso noted that low doses may still be effective and continue to work after the duration of active treatment.6 Although the FDA does not recommend once-daily dosing because the safety has not been confirmed, many of the panelists said that they prescribe isotretinoin once per day, particularly if the patient is taking a lower dose.6 The panelists also noted that once-daily dosing may be advantageous for patient adherence. Also if the patient’s morning meal is not high enough in fat to promote optimal pharmacokinetics, particularly if the patient is taking a generic form, then once-daily dosage later in the day may be preferable.
Disclosure
This Frontline Forum is supported by Sun Pharmaceutical Industries Ltd.
References
4. Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156(6):621-630. doi:10.1001/jamadermatol.2020.0465
5. Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18(5):412-418.
6. Accutane. Prescribing information. Roche; 2008. Accessed May 11, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/018662s059lbl.pdf
7. Roche stops selling acne drug Accutane. News release. June 26, 2009. Accessed May 11, 2023.https://www.reuters.com/article/us-roche-acne/roche-stops-selling-acne-drug-accutane-idUSTRE55P53C20090626
8. Accutane lawsuits: what’s happening now? Enjuris.com. 2023. Accessed May 11, 2023.https://www.enjuris.com/pharmaceutical-liability/accutane-lawsuits/#:~:text=Manufacturer%20Roche%20quietly%20removed%20Accutane,psychiatric%20conditions%20including%20suicide%20emerged






