- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Cosmetic procedure complications in darker skin
Author(s):
Some adverse reactions to cosmetic procedures occur more often in patients with darker skin types. Patients should be counseled on appropriate cosmetic procedures for their skin type, according to Cheryl M. Burgess, M.D., at the Skin of Color Update virtual meeting.
Whether or not dermatologists perform cosmetic procedures, it is important that they understand the spectrum of adverse reactions that can occur in patients with skin of color because they may see patients seeking care because of a com- plication, said Cheryl M. Burgess, M.D., at the Skin of Color Update virtual meeting. “With the advent of medical spas and the cross-over of various specialists into cosmetic procedures, I have seen an upsurge in the number of patients with Fitzpatrick skin types IV, V, and VI who come to me because they experienced an adverse event following a treatment involving a laser or light-based device, filler or neuromodulator injection, or chemical peel,” says Dr. Burgess, founder and president, Center for Dermatology and Dermatologic Surgery, Washington, D.C.
She described the types of reactions that are seen most often in skin of color and offered recommendations for minimizing risks and achieving patient satisfaction. Summarizing her top tips, Dr. Burgess says, “Careful patient selection is important for achieving the desired out- come with any cosmetic procedure, and thorough counseling should be provided at consultation on procedures that are appropriate for the individual’s skin type. Performing a test spot may be useful to determine potential adverse events with lasers and chemical peels, and laser parameters may need to be tweaked for increased safety.”
She emphasizes that it is best to start conservatively and recognize that achieving the desired outcome may require multiple sessions.
Laser and light systems
First and second-degree burns are among the most common complications seen in skin of color patients who undergo cosmetic procedures involving a laser, and they are particularly a problem with the use of intense pulsed light devices. The burns are easily recognized by their rectangular shape, Dr. Burgess says.
Postinflammatory hypopigmentation can occur after hair removal using the short pulse 1064-nm Q-switched Nd:YAG laser whereas hyperpigmentation was more common in the past when the procedure was performed with the 532-nm systems.
Patient with Fitzpatrick Skin Type V shown before with burns from IPL treatment and after treatment with 650 microsec Nd:Yag laser, 1927 nm fractionated thulium laser, chemical peels and triple cream.
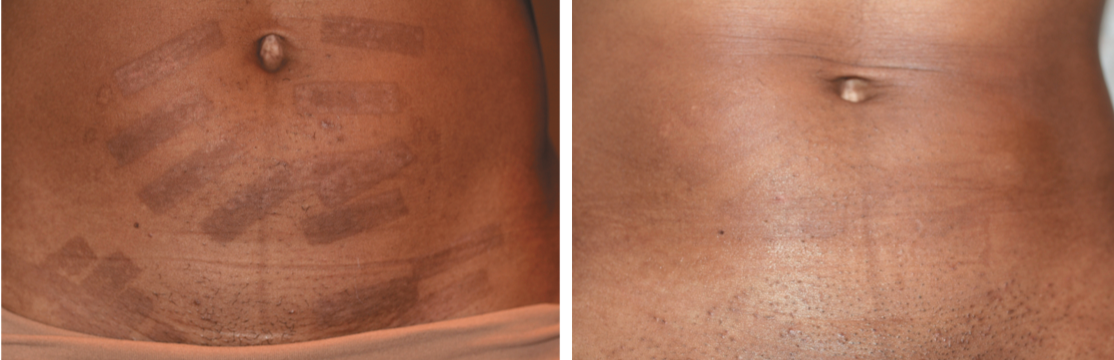
“The longer 1064-nm wavelength avoids hypopigmentary changes since it bypasses the melanin in the epidermis. Its absorption deeper in the hair leads to thermal destruction of the follicle, which makes the longer wave Nd:YAG laser ideal for hair removal in skin of color,” Dr. Burgess says.
Whenever performing a laser procedure in a new patient with skin of color, however, Dr. Burgess advises performing a test spot and using a lower fluence.
“Manufacturer-recommended parameters are based on clinical trials that enrolled mostly patients with Fitzpatrick skin types I-III and some patients with skin type IV, but rarely any- one with darker skin,” she says. “When patients understand the test is being done for their safety, they are willing to accept the delay.”
Filler reactions
Safety data from premarketing clinical trials with various fillers did not show any increased risk of pigmentary changes or hypertrophic or keloidal scarring in patients with skin of color, although the eligibility criteria for these studies excluded patients with a history of keloids. As in patients with lighter skin types, bruising is the most common adverse event associated with filler injections in skin of color. Hyperpigmentation can also occur, particularly with the use of a serial puncture technique and faster injections times.
“Filler injections in skin of color patients are best done slowly using a linear threading technique,” Dr. Burgess says.
Neuromodulator injections
Dr. Burgess notes that Asian patients, particularly, request treatment for crow’s feet and glabellar frown lines. Injections into the forehead and periocular region in these patients requires care to avoid the development of eyebrow or eyelid ptosis that will decrease the palpebral aperture.
“As people age and the laxity of the forehead and upper eyelid skin becomes more
apparent, a reduced dose or avoidance of botulinumtoxinA injections is recommended to avoid eyebrow ptosis,” Dr. Burgess says. Chemical peels
Superficial chemical peels using salicylic acid, lactic acid, malic acid, or glycolic acid can be performed safely in skin of color. The use of medium and deep peeling agents, however, risks dyschromia from rapid skin exfoliation that leads to a deeper exfoliation to the lower levels of the epidermis and melanocytes with subsequent inflammation and ultimately hyperpigmentation.
Dr. Burgess says that neutralization of the acid is a technique used in medical spas to minimize risk in skin of color. However, a neutral pH is no longer an acid strength to perform chemoexfoliation.
“Chemical peels at lower pH require observation of the skin’s reaction and erythema. The end point for a peel isn’t always ‘until it frosts’ in skin of color,” Dr. Burgess says
Disclosure:
Dr. Burgess receives honoraria, does clinical research for, and/or is on the advisory board for Allergan, Revance, Merz, and Prollenium.\






