- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Benzene Found in Benzoyl Peroxide-Containing Acne Products: What Comes Next?
Author(s):
From our April cover feature: Take an in-depth look at the basis of Valisure's benzene testing methods and practical recommendations for clinicians from Christopher Bunick, MD, PhD.
Credit: Valisure
Benzoyl peroxide (BPO) has been a hot topic in both dermatology and consumer headlines after news broke about high levels of benzene found in BPO-containing acne products such as cleansers, lotions, and gels. On March 6, 2024, Valisure LLC, an independent testing laboratory in Connecticut, filed a petition with the FDA requesting recalls of BPO products containing or forming high levels of benzene, a known human carcinogen.1
Valisure tested 66 BPO products and found high levels of benzene when BPO products were incubated at 37 °C (98.6 °F: body temperature), 50 °C (122 °F: accepted pharmaceutical stability testing temperature), and 70 °C (158 °F: hot car temperature).
Valisure’s findings have been criticized by some individuals, as questions were raised about testing products at such high temperatures. Valisure has been transparent about the reason for the temperatures in their stability testing, writing, “The purpose of stability studies is not to copy the conditions of an average consumer, rather, the purpose is to ensure the product is safe to use for the entire product’s lifecycle, from manufacturing through distribution to its expiration date, often a total of 3 years for pharmaceutical products. Products must be stable during this entire time and through all the possible ‘bumps’ along the road. It is common to condense the total stability of a product intended for multiple years of use by performing ‘accelerated’ stability studies that elevate the temperature for a few months. For example, according to publicly available calculators, 3 years of room temperature stability is equal to about 169 days at 50 °C (122 °F). Valisure broadly observed dramatically high levels of benzene in only 18 days at 50 °C.”2 The implication is that many BPO-containing products do not meet stability regulations.
Valisure also noted that the FDA specifically prefers this testing approach, which is outlined in its regulations on storage conditions.3
Christopher Bunick, MD, PhD, FAAD, an associate professor of dermatology and a physician-scientist at the Yale School of Medicine in New Haven, Connecticut, has closely followed benzene contamination news in dermatology since the first detection of benzene contamination in sunscreen in 2021. At the 2024 American Academy of Dermatology Annual Meeting in San Diego, California, Bunick presented updates on benzene in BPO to an overflowing room of attendees looking for more information and data. Bunick has no financial disclosures related to Valisure and is passionate about providing dermatologists with up-to-date information to help better inform their patients.4
Bunick began with a history of benzene through academic papers, starting with a German paper from 1936 that evaluated the thermal decomposition of diacyl peroxides, which includes BPO. In 1997, manufacturing company AkzoNobel filed for a patent for a method for the “reduction of benzene formation in dibenzoyl peroxide formulations.”
“Recently, however, it has been discovered that BPO formulations such as pastes, emulsions, and suspensions based on organic plasticizers, whether in the presence or absence of water, show continuously increasing levels of free benzene throughout their useful lifetime. This free benzene is formed due to the slow decomposition of the BPO over time,” AkzoNobel noted in its patent application. AkzoNobel also mentioned that the minimization of benzene’s formation is highly desirable due to its known carcinogenic properties.
“Almost 20 to 30 years ago, a company was trying to solve the benzoyl peroxide decomposition problem,” said Bunick.
Sixteen years before the AkzoNobel patent application for plastics, animal studies showed possible tumor formation, leading the FDA to complete an investigation where they did not fully deem BPO safe. After animal testing, it was found that “benzoyl peroxide is a tumor promoter in several animal species. The significance of this finding in humans is unknown,” a statement that is now added to the nonclinical toxicology section of a BPO-containing product’s package label.5
The US Pharmacopeia (USP) released a statement on March 8, 2024, related to the benzene testing from Valisure, stating that benzene contamination should be taken seriously, but that if changes are made to a USP method, complete validation of data is needed to prove a product meets USP standards.6
“Perhaps the most important line in this entire statement is: ‘It is important to note that USP standards are applicable during the entire shelf life of the product, not just at the time of release,’” said Bunick. “The problem with the USP statement is that the FDA already rejected their methods as not being good enough, and you wouldn’t know that unless you read the FDA’s statement.”
In 2020, the FDA rejected USP’s method of gas chromatography–flame ionization detection for benzene analysis, which is a method that has less precision and accuracy.7
The FDA elected the industry standard of gas chromatography–mass spectrometry (GC-MS), which is more accurate, and is the method Valisure chose to use in its recent petition.
Validating Valisure’s Findings

The findings of benzene in BPO products were independently validated by 3 different labs using 3 different technology platforms, including GC-MS, GC–high-resolution mass spectrometry, and selected-ion flow-tube mass spectrometry. Bunick shared updates that CBS News followed up with the labs, and 2 of the 3 responded and confirmed Valisure’s results when contacted.
“There have been concerns that, superficially, when you look at the petition, that it’s all about elevated temperatures. That is not true. Benzene was also found extensively at room temperature on the shelf and off the shelf. This important fact cannot be overlooked. The focus of the petition is stability studies,” said Bunick.
Of the 66 products tested at day 0 at room temperature, 10 of the products had more than 10 ppm of benzene and 19 products had more than 2 ppm of benzene. According to Bunick, the biggest problem with BPO is degradation, not contamination. He also reminded attendees that although the FDA permits up to 2 ppm of benzene, that is only if it is absolutely necessary to manufacture said product and that product represents a major medical advance. Bunick added that with so many other acne treatments available, it is not absolutely necessary to manufacture BPO with unsafe levels of benzene, even at 2 ppm.
Stability testing is not meant to replicate consumer use or real-world use; it is intended to evaluate the full 3-year product life cycle. Three-year stability at room temperature is equal to 169 days at 50 °C (122 °F), which is based on industry standards. It is a regulatory requirement to perform stability testing on products and should be conducted on products under normal storage conditions, or, preferably, under exaggerated conditions, according to the FDA.
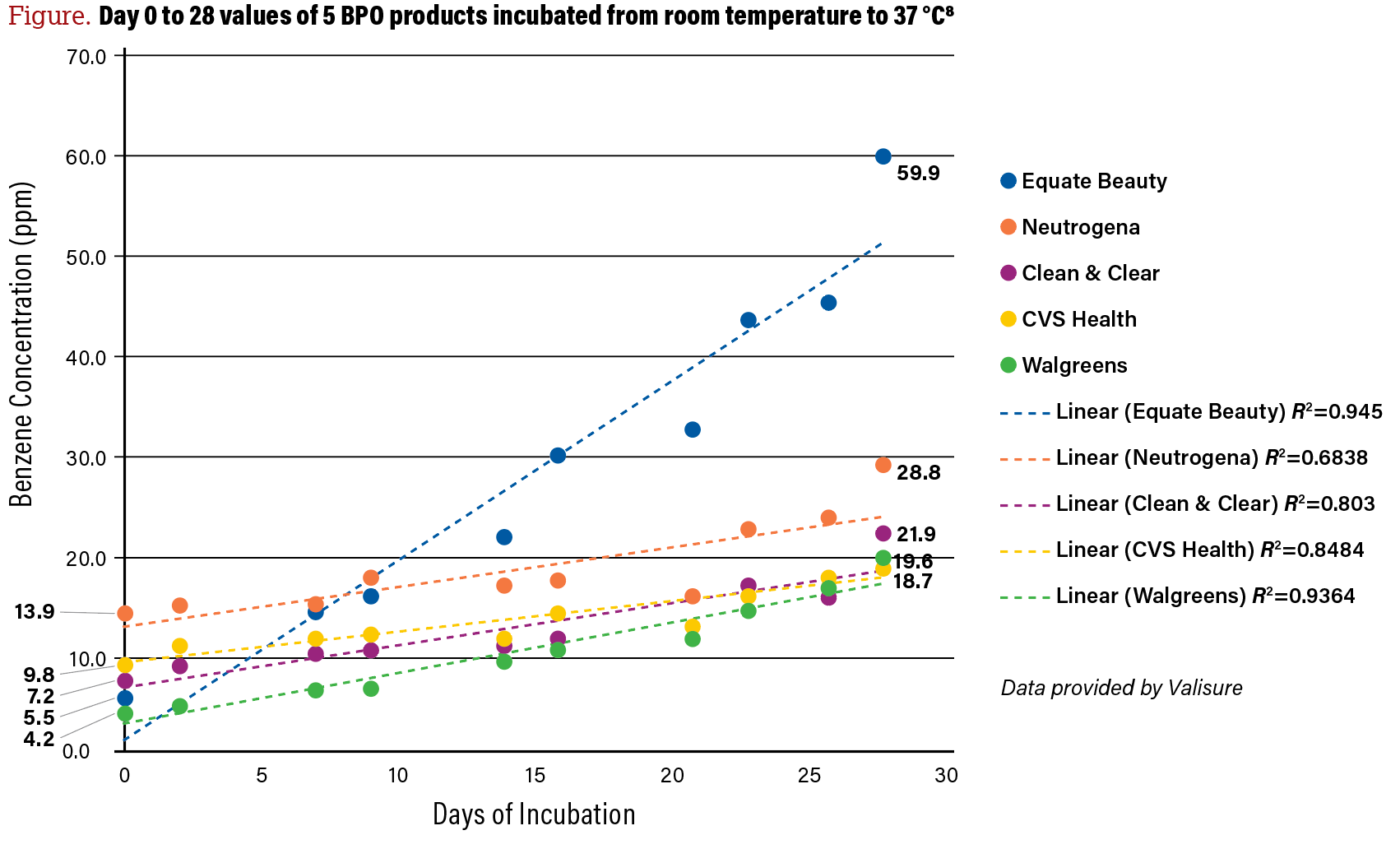
However, in some cases, stability testing does overlap with real-world outcomes. Bunick shared new data for the first time at AAD 2024 that demonstrated that of 5 BPO products tested at 37 °C (98.6 °F), or human body temperature, 1 product had more than 10 ppm of benzene, and all 5 had over 2 ppm of benzene. This 37 °C data was published shortly after the AAD in a peer-reviewed journal, Environmental Health Perspectives; Bunick is a co-author along with the Valisure research team (Figure).8
Figure. Day 0 to 28 values of 5 BPO products incubated from room temperature to 37 °C8
Credit: Valisure
When considering Valisure’s testing, Bunick pointed out that every time Valisure has detected a carcinogen in consumer products, some form of a recall followed.
Bunick noted that some individuals and organizations have questioned Valisure and its credibility. Valisure is a company hired by the US Department of Defense to check the quality of drugs for the United States military. Their No. 1 clients are drug purchasers, according to Bunick, because these companies want to know what they are buying is safe.
Lastly, Bunick concluded his session by reviewing recent benzene studies and news items, all of which called out benzene’s associated risk with various cancers such as leukemia. Regulatory agencies such as the CDC, the World Health Organization, the Environmental Protection Agency, and the National Institute for Occupational Safety and Health have all made similar statements that benzene is a known human carcinogen.
Additionally, in December 2023, the FDA released new guidance for industry for the reformulation of drug products containing carbomers manufactured with benzene.9

“So, what does this all mean, and what am I going to advise for patients? I will say, we as a community have to see what is validated.I do not necessarily think at this moment that no one should use benzoyl peroxide. I think there has to be a proportional response until more data comes out,” said Bunick.
“With Valisure’s agreement, for those providers and patients who wish to continue using benzoyl peroxide–containing products, a proportional response at this time would be to keep the product refrigerated at all times, renew the medicine every 3 to 6 months, and avoid heated storage. This will not necessarily eliminate all benzene but should slow its decomposition. This measure gives the medical community a chance to await further investigation and recommendations,” Bunick told Dermatology Times.
References
1. Andrus E, Bader K. Benzene found in various acne products; Valisure files petition with FDA to recall treatments. Dermatology Times. March 6, 2024. Accessed March 14, 2024. https://www.dermatologytimes.com/view/breaking-news-benzene-found-in-various-acne-products-valisure-files-petition-with-fda-to-recall-treatments
2. What is “stability testing” & why it’s important. News release. Valisure. March 7, 2024. Accessed March 14, 2024. https://www.valisure.com/valisure-newsroom/the-importance-of-stability-testing
3. FDA. Expiration dating and stability testing for human drug products. Updated November 7, 2014. Accessed March 14, 2024. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/expiration-dating-and-stability-testing-human-drug-products
4. Bunick CG. Discussion of new findings of benzene in BPO-containing products. Presented at: 2024 American Academy of Dermatology Annual Meeting; March 8-12, 2024; San Diego, CA.
5. Epiduo. Prescribing information. Galderma; 2011. Accessed March 19, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022320s001lbl.pdf
6. USP statement on third party laboratory benzene findings. News release. USP. March 8, 2024. Accessed March 14, 2024. https://www.usp.org/news/statement-on-third-party-laboratory-benzene-findings
7. FDA. Direct injection gas chromatography mass spectrometry (GC-MS) method for the detection of listed impurities in hand sanitizers. August 24, 2020. Accessed March 14, 2024. https://www.fda.gov/media/141501/download
8. Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132(3):37702. doi:10.1289/EHP13984
9. FDA. Reformulating drug products that contain carbomers manufactured with benzene: guidance for industry. December 27, 2023. Accessed March 14, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ reformulating-drug-products-contain-carbomers- manufactured-benzene






