- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Administrative Burden of Prescribing Biologics
Author(s):
Prescribing biologics can require a complex approval and claims process. Creating a tiered system based on office workload can help practices understand and allocate staff time. Outsourcing approvals to pharmacies where possible can also streamline the process.
With the constant advances in the field of dermatology, biologic therapies are quickly becoming a mainstay in treatment for many dermatology patients. With the increased numbers of biologic therapies utilized on a daily basis, the administrative burden and growing frustrations of patients are becoming evident. There is a need for a more streamlined process with biologic medication approvals, and the collaboration with specialty pharmacies and pharmaceutical companies is essential. The following paper outlines these burdens and proposes a framework in 6 steps for minimizing the administrative burdens in a private practice dermatology clinic.
Step 1: Evaluation
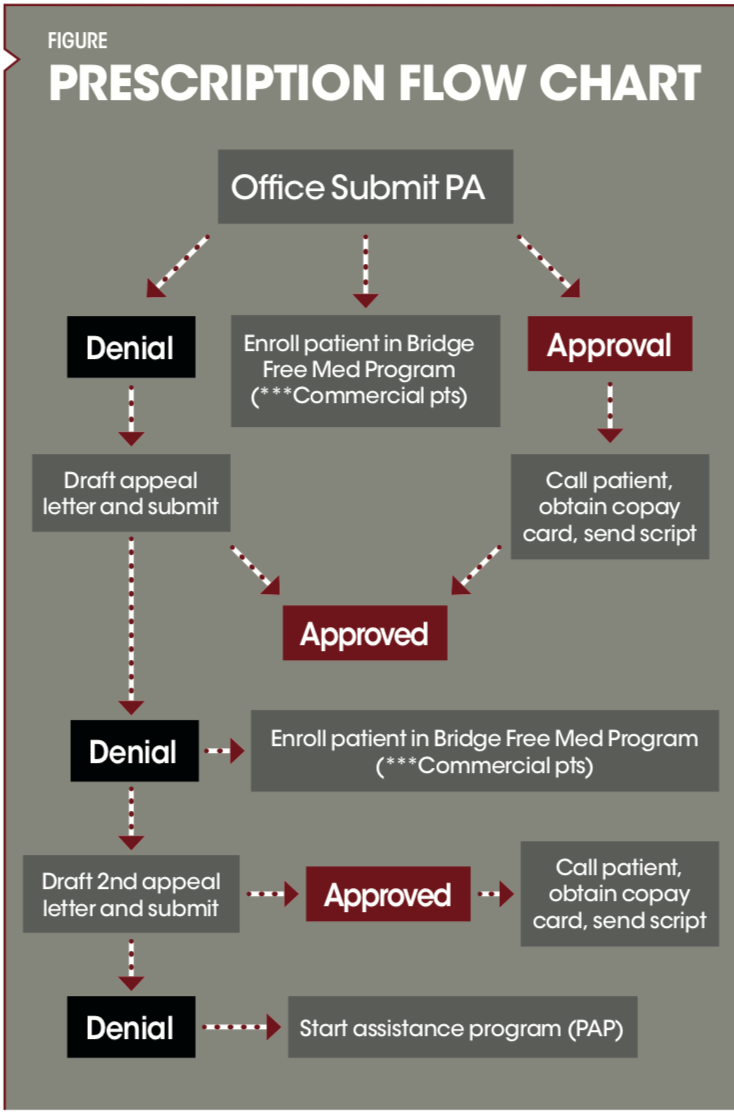
We evaluated the process from the time the prescriber writes a prescription for a biologic to the time the patient receives the medication, and created a flow chart of the entire process.
We then identified problems and concerns throughout the process for our staff and our patients. Prior authorizations require added work for our employees, and denials can lead to a long appeal process for the patient. Many manufacturers offer free drug or bridge programs for commercially insured patients. Each manufacturer has specific requirements as to when the patient can be enrolled in these programs. Some specialty pharmacies are available to assist the practice with this process, including enrollment into free drug programs; however, some medications are excluded depending on the specialty pharmacy used. For excluded medications, the burden of prior authorization submission, appeals, and follow-up fall on the practice.
Step 2: Creating a Hierarchy of Biologics
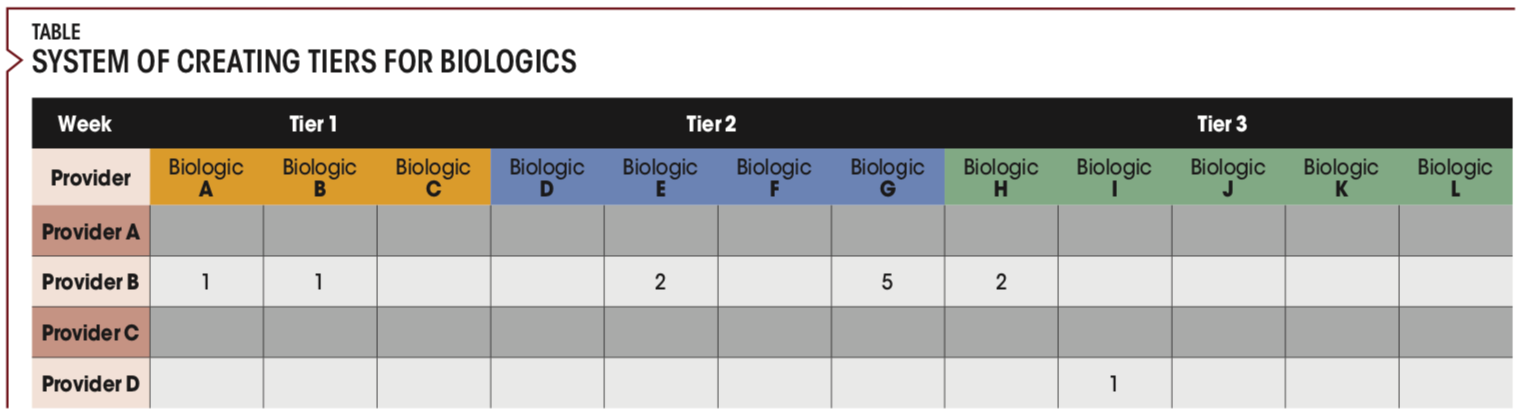
The administrative team stratified biologic medications based on office workload requirements for medication approval. Biologics are categorized into a tiered system (Tiers 1-3).
Tier 1
Tier 1 medications require the least amount of work for the practice. These are medications that can be sent to a specialty pharmacy. Tier 1 medications have a good free drug or bridge program, which minimizes delays in the approval process. All the practice needs to do is submit the prescription to the specialty pharmacy, which handles the prior authorizations, appeals, and free drug programs entirely for the practice. Medications with free drug programs that the specialty pharmacy is unable to facilitate are not included in Tier 1.
Tier 2
Tier 2 medications can also be sent to a specialty pharmacy, but because of pharmaceutical company restrictions, the specialty pharmacy is unable to enroll the patient in free drug programs on the practice’s behalf. This usually delays approval for the patient and creates additional work for the practice.
Tier 3
Because of restrictions from the pharmaceutical companies, specialty pharmacies are unable to assist with Tier 3 medications.
System of creating tiers for biologics
Step 3: Educating Providers
Providers were educated on the aforementioned steps. The differentiation and workload requirements for Tier 1 through Tier 3 medications were explained. While providers are at liberty to prescribe any medication, they were encouraged to keep this tier system in mind when doing so. Prescribers are updated weekly with prescribing data using the chart below.
Prescription flow chart
Step 4: Creating and distributing an informational handout for patients
As we are using a specialty pharmacy to assist in the prior authorization process, we wanted to direct the patients to the pharmacy for all of their prescription-related questions and concerns. We created a half-sheet with contact information for the specialty pharmacy as well as a list of Tier 1 and Tier 2 drugs. Our goal was to have the patient contact the specialty pharmacy directly in order to take the burden of callbacks off our staff. The Tier 1 drugs were highlighted in yellow in hopes of reminding providers to prescribe Tier 1 medications when appropriate.
Step 5: Communicating to Pharmaceutical Representatives
Pharmaceutical representatives for each company were informed of their medication’s representation on our hierarchy chart. For medications in Tier 2 and Tier 3, we explained the hurdles that we are facing in the approval process of their medication. We encouraged the pharmaceutical representatives to collaborate with our specialty pharmacy to improve the process and alleviate some of the challenges faced by both the patients and our office. When pharmaceutical representatives provide potential solutions, these solutions are vetted before being fully implemented, changing the hierarchy, and communicating to providers.
Step 6: Discussions with Specialty Pharmacies
We communicated with current and potential specialty pharmacy partners to maximize efficiency in the approval process of biologics. Delays in the approval process were identified including requests for information via fax and phone to complete the prior authorizations and/or appeals. We are able decrease calls and fax requests from the pharmacies by signing non-disclosure agreements and/or business associate agreements, which allowed them to have direct access to our electronic medical record system. This allows our pharmacy colleagues to have access to test results, tried and failed medications, and detailed patient history.
Conclusion
The recent systematic evaluation of biologic prescribing and approval revealed numerous burdens for both the patient and the office. The new program implemented at our office aims to provide a more streamlined approval process. The ultimate goal is to achieve an efficient system that provides the best experience for both the patient and our employees. A collaborative approach with support from specialty pharmacies and pharmaceutical companies appears critical.
Disclosures:
Funk, Reedy and Torok report no relevant or financial disclosures.







