- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
News
Article
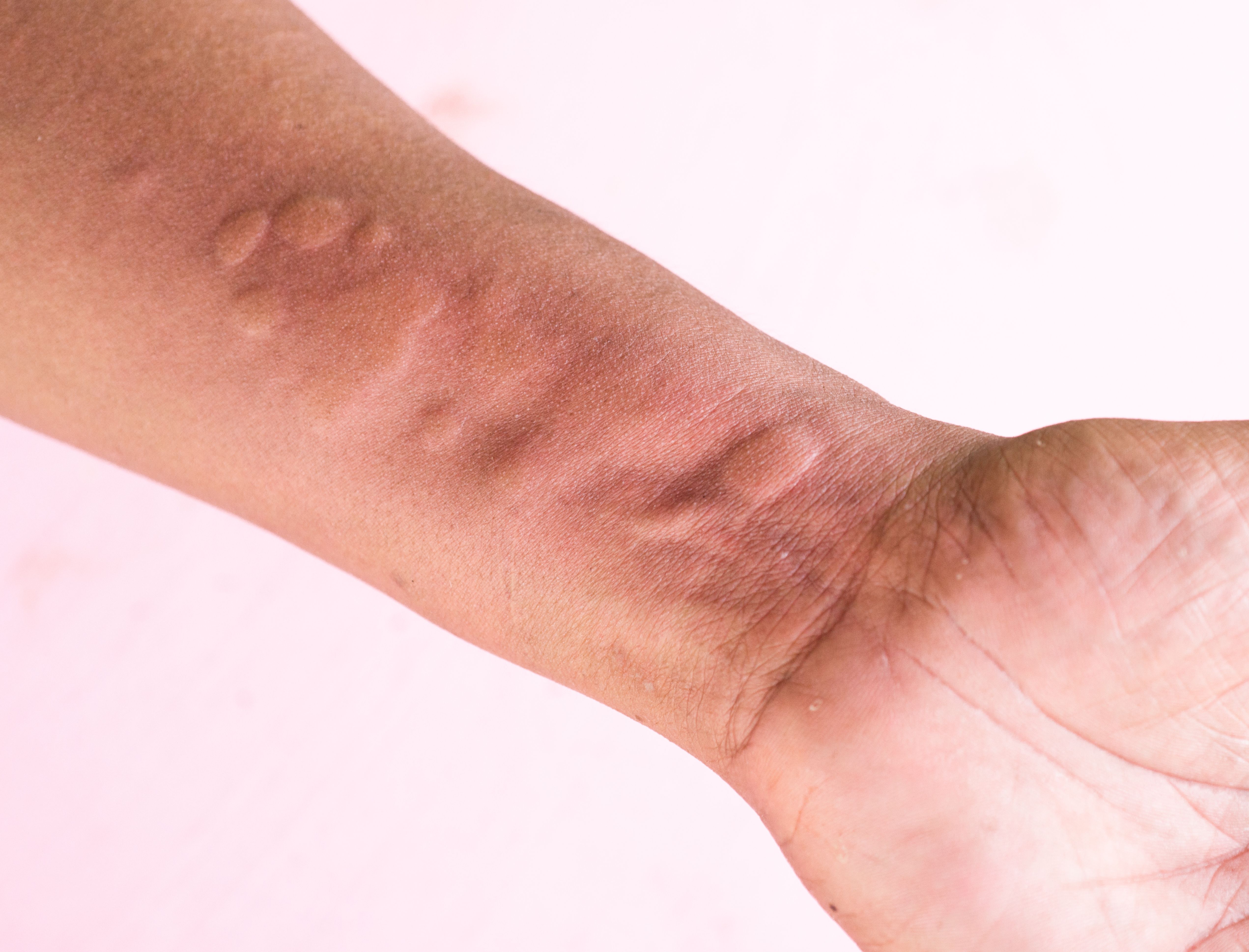
A Deep Dive Into the Chronic Spontaneous Urticaria Armamentarium
Author(s):
Dawn Merritt, DO, and Mark Lebwohl, MD, took the stage to explain the history of CSU, the prevalence of testing and food avoidance, approved treatments, and monitoring treatment improvement for the condition.
“Only 6% of dermatologists report prescribing omalizumab to patients with chronic spontaneous urticaria, and there’s opportunity for dermatologists to take back ownership of the disease,” Dawn Merritt, DO said in the session “What Dermatologists Need to Know About Chronic Spontaneous Urticaria (CSU) and the Role of Biologics” at the 2023 Fall Clinical Dermatology Conference in Las Vegas, Nevada October 19-22.
She and Mark Lebwohl, MD,took the stage to explain the history of CSU, the prevalence of testing and food avoidance, approved treatments, and monitoring treatment improvement for the condition. Merritt serves as the dermatology residency program director at Ohio Health O’Bleness Hospital in Athens, Ohio. Lebwohl is a Dermatology Times Editorial Advisory Board member and the dean for clinical therapies and chairman emeritus at the Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine at Mount Sinai in New York, New York.
Natural History of CSU
Many patients with CSU do not make it to a dermatology clinician because about half will go into remission without therapy within the first 12 months.The other half of patients may be waiting a while before getting answers to their wheals and angioedemas. Diagnostic delays are common with CSU and contribute to the disease duration and burden. In fact, 11% of patients will have symptoms for more than 5 years before diagnosis.2
Routine Laboratory Testing: Is It Needed?
Merritt and Lebwohl explained that laboratory testing to diagnose CSU is not ideal and referred to a study showing that out of 1872 tests ran among 356 patients with CSU, only 1 test resulted in a management change in 1 patient.3
“There is no lab test that makes a diagnosis of CSU, not a blood test. If a patient comes in with hives, I can usually tell right away and the question I ask them is ‘how long do they stay in one place?’ If they move around over hours that's urticaria, that's what it means.” said Lebwohl. “And on the other hand, if the patient comes in and says ‘you know, I've got these marks but they're gone.’There's nothing when you see them, what do you do? You scratch their arm, and if they get dermal graphism and they're describing red lesions that come and go, that's urticaria. It'sa really easy diagnosis.”
The prevalence of food allergies and relevant avoidance was researched in China. 32% of patients with CSU avoided fish, shrimp, lamb, crab, or beef. 22% were positive for food-specificIgE, but it did not correlate with avoiding foods. The study pointed out that CSU caused by food allergy was less than 3%.4
Treatment Options
Guidelines to treat CSU have not been updated in the US since 2014. European guidelines were updated in 2021 to recommend cyclosporin up to 5mg/kg, and if that is not showing response, a dose of omalizumab can be increased to 600mg every 2 weeks.5
The treatment landscape for CSU includes doxepin (Sinequan), leukotriene modifiers, corticosteroids, immunosuppressive agents, dapsone, hydroxychloroquine (Plaquenil), and omalizumab (Xolair).
Merritt and Lebwohl took time to praise the approval of omalizumab for treatment in 2014. Despite nearly a decade of availability and strong efficacy data (80%), only 6% of dermatologists prescribe it. Originally the drug required office injections. In April 2021, home-based injections were approved. The lack of use stems from the box warning of anaphylaxis. Merrit and Lebwohl showed anaphylaxis incidence data in relation to all biologics and the risk with omalizumab was lower than many common biologics prescribed daily.6
There are additional CSU drugs in the pipeline including dupilumab (Liberty-Cupid phase 3), remibrutinib (Remix 1 & Remix 2 phase 3), fenebrutinib (NCT03137069 phase 2), and tezepelumab (Inception phase 2).
Monitoring CSU Treatment Progress
The Urticaria Control Test (UCT) is an assessment Merrittutilizes at each appointment with a CSU patienttomonitor a treatment plan’s progress. It consists of 4 questions about CSU symptoms, quality of life, treatment frequency, and whether the patient has had their uticaria under control over the last 4 weeks. The results can total up to 16 points. Merritt says that any score below 12 needs to be examined closely for additional considerations in treatment strategies. She also concluded with guidance to help a patient once their CSU is under control.7
“As you want the patient to be completely controlled on omalizumab on whatever dose you're on. Hold them for somewhere at least 9 to 12 months. So you're looking for a really long period of control. And then you're going to slowly back them down to get to 150 milligrams, which is 1 injection and at that point, you're going to start spreading out their injections,” she explained. “You'll go from 4 weeks to 6 weeks. When you get down to 150 milligrams, then you can safely take them off. If at any point they relapsed. You go back to the dose and you back up. The good news is there's really good recapture data with omalizumab.”
References
- Merritt D, Lebwohl M. What Dermatologists Need to Know About Chronic Spontaneous Urticaria and the Role of Biologics. Presented at: 2023 Fall Clinical Dermatology Conference; October 19-22, 2023, Las Vegas, NV.
- Antia C, Baquerizo K, Korman A, Bernstein JA, Alikhan A. Urticaria: A comprehensive review: Epidemiology, diagnosis, and work-up. J Am Acad Dermatol. 2018;79(4):599-614. doi:10.1016/j.jaad.2018.01.020
- Tarbox JA, Gutta RC, Radojicic C, Lang DM. Utility of routine laboratory testing in management of chronic urticaria/angioedema. Ann Allergy Asthma Immunol. 2011;107(3):239-243. doi:10.1016/j.anai.2011.06.008
- Hsu ML, Li LF. Prevalence of food avoidance and food allergy in Chinese patients with chronic urticaria. Br J Dermatol. 2012;166(4):747-752. doi:10.1111/j.1365-2133.2011.10733.x
- Zuberbier T, Bernstein JA, Maurer M. Chronic spontaneous urticaria guidelines: What is new? [published correction appears in J Allergy Clin Immunol. 2023 Feb;151(2):580]. J Allergy Clin Immunol. 2022;150(6):1249-1255. doi:10.1016/j.jaci.2022.10.004
- Bian S, Zhang P, Li L, et al. Anaphylaxis Associated With Allergen Specific Immunotherapy, Omalizumab, and Dupilumab: A Real World Study Based on the US Food and Drug Administration Adverse Event Reporting System. Front Pharmacol. 2021;12:767999. Published 2021 Oct 22. doi:10.3389/fphar.2021.767999
- Weller K, Groffik A, Church MK, et al. Development and validation of the Urticaria Control Test: a patient-reported outcome instrument for assessing urticaria control. J Allergy Clin Immunol. 2014;133(5):1365-1372.e13726. doi:10.1016/j.jaci.2013.12.1076