- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Verrica develops a solution for common warts
Author(s):
Dr. Matt Davidson, Ph.D., wanted to find an effective and affortable treatment for the recurring common warts on his hands, which has lead him to become one of the youngest, if not youngest, pharmaceutical company pioneers in the country.
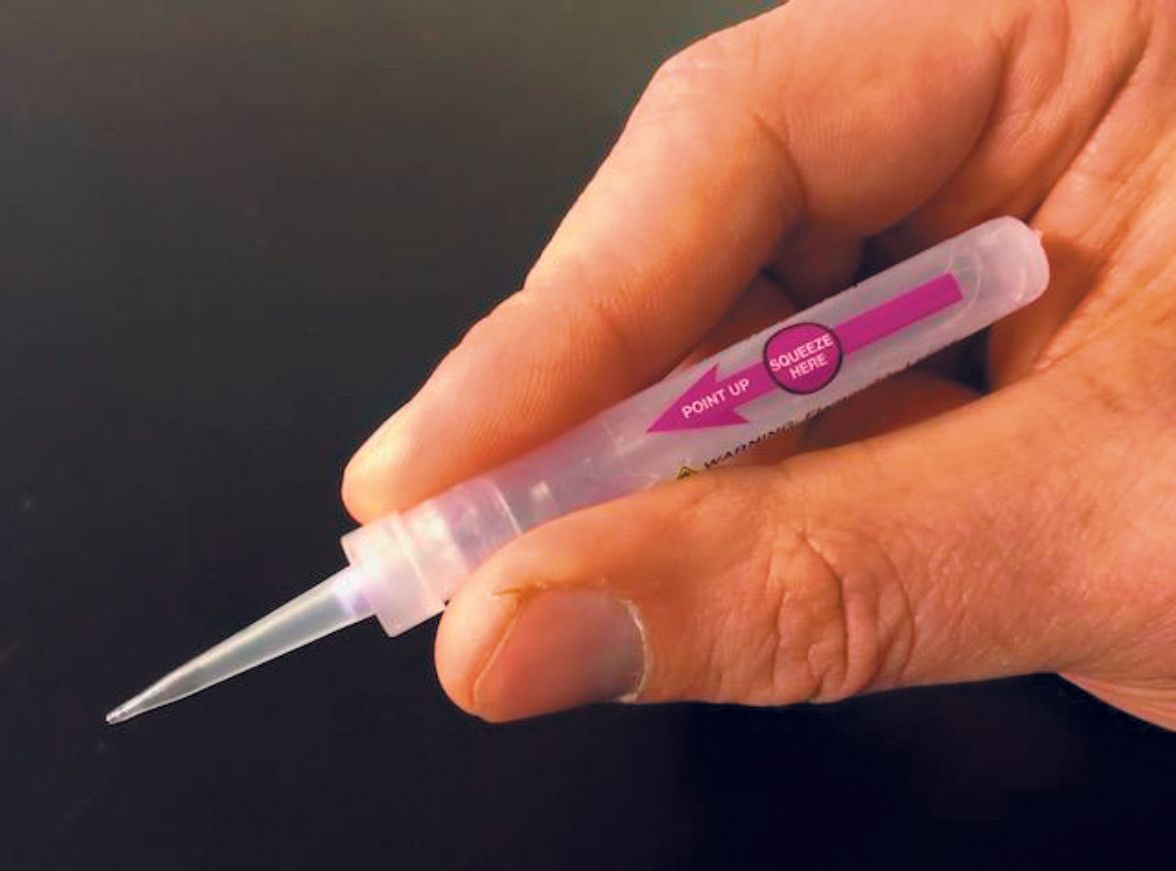
Verrica’s lead product candidate, VP-102, is administered via a single-use, precision applicator. (Photo courtesy of Verrica Pharmaceuticals.)
Dr. Davidson

Mr. White

Verrica Pharmaceuticals went public in June 2018 raising over $86 million in its first public stock offering. The company’s founder is Matt Davidson, Ph.D., who laid the groundwork for the company from his garage shortly after graduating from Stanford Unviversity. Photo courtesy of Verrica Pharmaceuticals.

As a young scientist transitioning from graduate school to a career in the pharmaceutical industry, Matt Davidson, Ph.D., knows how important it is to make a good first impression.
But ever since childhood, he struggled with recurring common warts on his hands.
“He was embarrassed to shake other people’s hands,” said Ted White, Verrica’s CEO and president. So, like any scientist, Dr. Davidson began pouring over the science.
“The literature was compelling. I read papers on cantharidin going back to the 1960s. John and William Epstein published a key paper in 1960, but it was a short paper only two pages long that described a study using a 0.7 percent concentration to treat warts on fingers,” Dr. Davidson said. He was able to secure a non-FDA approved version of cantharidin which cleared his skin. His personal success with cantharidin and the existing literature on cantharidin, “got me interested in pursuing this line of research,” he said.
After graduation, Dr. Davidson worked for Applied Immunology (now Precision for Medicine), but at night, he was busy laying the groundwork for Verrica from his garage at his home in the San Francisco Bay Area. Here, he met with mentors and advisors who had successfully launched products or companies: Dennis Brown, Ph.D., of Matrix Pharmaceuticals; Mark de Souza, Ph.D., of Lotus Tissue Repair; and, Glenn Oclassen of Oclassen Pharmaceuticals.
“I had really great mentors who served as advisors early on. They had all founded and successfully ran companies in the dermatology space,” he said.
Now 34, Dr. Davidson is one of the youngest, if not youngest, pharmaceutical company pioneers in the country. As a student, he went through rounds of treatment with liquid nitrogen therapy (a painful procedure in which the lesions shrink but recur). He was eventually treated with cantharidin, which worked. And, so was born his desire to find an effective, affordable treatment with few-if any-side effects.
Headquartered in West Chester, Penn., Verrica specializes in the development of treatment for skin conditions, such as molluscum contagiosum and common warts. The active ingredient for its lead product candidate, VP-102, is the compound cantharidin. It is administered in solution through a single-use, precision applicator and is currently in clinical trials with results of two phase three clinical studies expected early next year.
The company name is based on a blend of the Latin name for warts (verruca) and the word “veracity.” “I like the idea of ‘veracity,’ in doing things the right way and being honest and forthcoming about it,” Dr. Davidson said.
VERRUCA VULGARIS
Currently, there is no FDA-approved treatment for verruca vulgaris, despite affecting 10-20 percent of the population.
In some cases, verruca vulgaris heals on its own, but in other cases, it may require cryotherapy, cauterization, tissue scraping, laser treatment, electrosurgery, among other treatments.
Cantharidin is the purified active ingredient of cantharides, which is dried, powdered blister beetles (also called Spanish flies). It has been documented in scientific studies as a possible treatment for warts since 1958.
Despite the lack of FDA approval, topical cantharidin has been used by patients for skin conditions, such as common warts, molluscum contagiosum, periungual warts, plantar warts, calluses, cutaneous leishmaniasis, herpes zoster, and acquired perforating dermatosis.
“Mylabris, the dried body of the Chinese blister beetle, has been used medicinally for more than 2,000 years in China and is still used as a folk medicine today in Asia,” according to a 2001 article published in JAMA by Mary Wu Chang, M.D., and colleagues (DOI:10.1001/archderm.137.10.1357)
Cantharidin was first documented in literature in the 1800s and was used by dermatologists to treat molluscum contagiosum and warts, but in 1962, the U.S. Food and Drug Administration changed the rules now requiring clinical trials to support efficacy claims. Today, the FDA tolerates the use of physician or pharmacist-compounded drug products that include cantharidin. The FDA is currently considering adding cantharidin to its bulk drug substance list for pharmacy compounding under Section 503A.
Cantharidin works by being absorbed into the lipid layers of epidermal cell membranes, which activates or releases neutral serine proteases that degenerate desmosmal plaque, “leading to detachment of tonofilaments from desmosomes,” write Dr. Chang and colleagues in JAMA (DOI:10.1001/archderm.137.10.1357). “This process leads to acantholysis and intraepidermal blistering, and nonspecific lysis of skin. Lesions heal without scarring, as acantholysis is intraepidermal.”
If used improperly, such as for consumption, it can cause poisoning. But when it’s used as instructed, “complications are exceedingly rare,” they wrote.
Without an FDA-approved cantharidin product, physicians are reluctant to use it in clinic due to the potential of problems associated with reimbursement issues and possible liability, Dr. Davidson said.
“So, we have an unmet need and a product that appears to be a good solution, but there is no mechanism for patients to access the treatment, which is when, in around 2012, I began to develop cantharidin in a quality-controlled manner under good manufacturing conditions,” he said.
In 2015, Dr. Davidson partnered with PBM Capital Group, a Charlottesville, Virginia-based healthcare investment group, to fund Verrica. "While common warts is an important indication, we decided that there was a greater need in the treatment of a similar pediatric viral skin disease called molluscum contagiosum,” he said.
“And then it was off to the races. With financing in place, I hired a chief medical officer, a head of clinical operations, got the drug manufactured at scale under good manufacturing conditions and the FDA allowed the start of clinical trials. Within five years, we went from inception to pivotal phase three trials,” he said.
DRUG DEVELOPMENT
In developing its cantharidin treatment, Verrica had to address a number of potential problems: access to ingredients, quality control and application. Previous trials, Dr. Davidson said, failed at the point of application. The Verrica team settled on an applicator modeled after a super glue applicator.
“Previous formulations were lacking in a workable, user-friendly formulation. Historically, people would take a jar of cantharidin specially formulated for themselves and then apply the treatment with a toothpick. Plus, they used the same jar repeatedly, which compromised the concentration of the product. It may start as a 0.7 percent solution, but it would very quickly dry out increasing in potency possibly leading to adverse events. Plus, it was frustrating because practitioners didn’t know how much to apply or how long to leave it on the skin,” he said.
The company purified cantharidin to greater than 99 percent, and formulated it in a film-forming solution within a single-use sealed glass ampoule. The clinician simply squeezes the applicator, which causes the glass to break. The solution then descends into the applicatortip, where a filter captures any glass particles.
The solution also includes small amounts of a purple surgical dye. “A faint violet color is placed on each lesion, so the provider knows which lesions has been treated,” White explains.
The first clinical trial of VP-102 for molluscum contagiosum was conducted in collaboration with Steven R. Cohen, M.D., M.P.H., chief if dermatology at the Albert Einstein College of Medicine, Bronx, N.Y. Patients received up to four treatments every three weeks. The treatment was shown to be safe and 44 percent of patients achieved complete lesion clearance by the 12-week endpoint with a mean lesion count reduction of 70 percent.
In a second phase two study with Verrica’s proprietary applicator, 50 percent of patients achieved completed clearance by the 12-week endpoint with the median patient clearing of 98 percent of lesions.
The two ongoing phase three trials compare the product to placebo because there are no FDA-approved treatments. No treatment-related serious adverse events have been reported in any of the company’s clinical studies.
When Verrica went public in June, it raised over $86 million. “We are looking for products that are unique, differentiated, have a patent life and are reimbursed by payers,” White said. “We are also truly excited that our current product is a new chemical entity (NCE) because there are not many NCEs in dermatology. Dermatology tends to be a lot of reformulations, like from a cream to a lotion or from lotion to ointment.”
“Verrica is always looking to work with practitioners who have identified unmet needs in dermatology, ,” Dr. Davidson said.
Verrica is also in a phase two trial for common warts using the same drug device product. And, next year, a phase two clinical trial for plantar warts is expected to commence. “Because plantar warts are much tougher to treat, we will use a more potent formulation of cantharidin and perhaps a different applicator,” White says.
“If our trials are successful, we expect to be the first FDA-approved therapy for the treatment of molluscum contagiosum and anticipate becoming the standard of care,” White said.







