- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Re-think rescue approach in atopic dermatitis
Author(s):
Dermatologists should not depend on a rescue approach for atopic dermatitis flares, says a recent paper. Instead, they should talk with patients about newer treatment options that could offer long-term disease with minimal adverse events.
“The time has come for clinicians treating [atopic dermatitis] to consider moving from a rescue approach for flares to treating [atopic dermatitis] as a chronic, inflammatory, cutaneous and systemic disorder..." says James Q. Del Rosso, D.O.
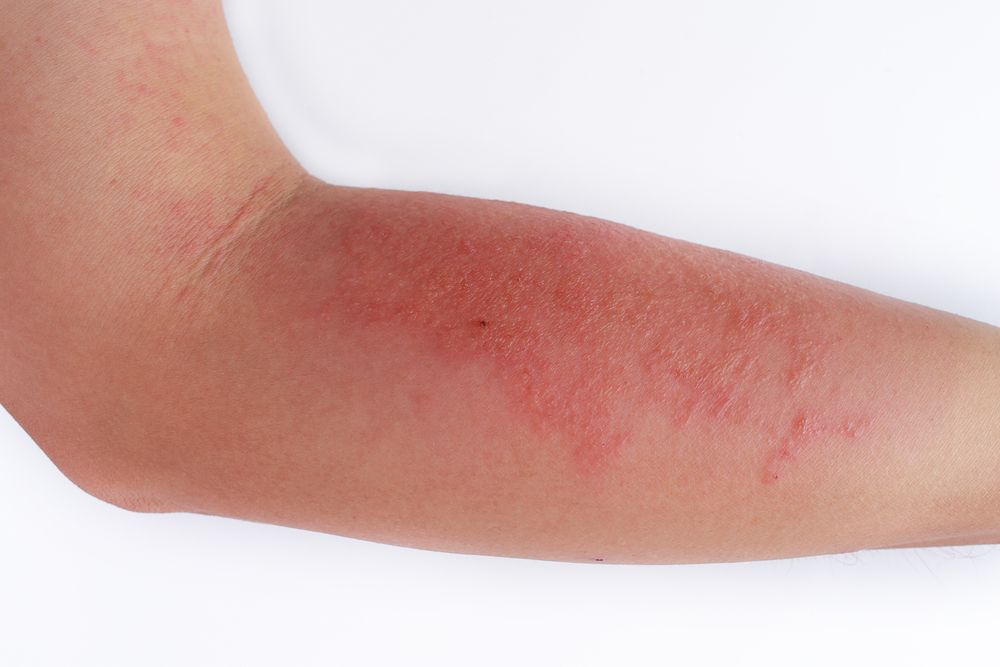
Dermatologists treating moderate-to-severe atopic dermatitis patients should not depend on a rescue approach aimed at responding to atopic dermatitis flares. Rather, clinicians should talk with patients about treatment options that could more optimally control symptoms in the long-term, while avoiding sometimes severe adverse events associated with conventional treatments, according to a paper published February 2019 in the Journal of Clinical and Aesthetic Dermatology.
Specifically, today’s dermatologists should consider using the injectable human IgG4 monoclonal antibody, dupilumab. Other monoclonal antibody IL-13 inhibitors, lebrikizumab and tralokinumab, are in the pipeline and could be among the future options for better controlling atopic dermatitis, according to the paper.
RELATED: Atopic dermatitis treatment advances on psoriasis research
“The time has come for clinicians treating [atopic dermatitis] to consider moving from a rescue approach for flares to treating [atopic dermatitis] as a chronic, inflammatory, cutaneous and systemic disorder by using therapies that more selectively suppress the underlying disease pathophysiology, effectively treat eczema and pruritus, mitigate flares and sustain long-term control of the disease,” writes the paper’s author James Q. Del Rosso, D.O., research director of JDR Dermatology Research in Las Vegas.
In the same manner that biologics for psoriasis and psoriatic arthritis have changed the set point for treatment response from partial to complete clearance, physicians and patients are at the precipice of a revolution of new biologics for atopic dermatitis, says Harry Dao, Jr., M.D., assistant professor of dermatology at Baylor College of Medicine, Houston, Texas.
Evolving spectrum of disease management
Conventional oral systemic treatments for atopic dermatitis and biologics might help patients, but treatment-especially long-term treatment-comes at the cost of sometimes serious adverse events.
Cyclosporine, methotrexate, azathioprine and mycophenolate mofetil seem to modulate pathophysiologic pathways that contribute to atopic dermatitis. And, while none are FDA approved for atopic dermatitis treatment, there’s data on each treatment in adults and children with the disease, Dr. Del Rosso writes.
The problem is these treatments tend not to have a good safety profile with long-term use.
“What is definitely known … is that we can avoid the immunosuppression and concomitant increased risk of cancer and infection in those treated with methotrexate, cyclosporine, azathioprine, and mycophenolate mofetil. These traditional immunosuppressive agents non-specifically lower an individual’s immune status and can prove toxic to various organs, especially with long-term use,” says Dr. Dao, who is not an author of the paper.
While dermatologists might use cyclosporine to treat resistant or uncontrolled atopic dermatitis, they usually transition patients to a safer treatment with time because continuous cyclosporine use is not recommended beyond 12 to 24 months, according to the paper.
Methotrexate, while it can start to relieve atopic dermatitis symptoms in as little as 4 to 8 weeks, also comes with what can be serious adverse events. Dermatologists might be less inclined to treat atopic dermatitis patients with azathioprine because of its slower onset and potential toxicities. Mycophenolate mofetil does not have quite the amount of data as cyclosporine but it appears to be the safest of these oral agents and offers an efficacy onset range of 4 to 12 weeks, according to the paper.
Potential of biologics
Researchers are studying many injectable and oral biologic options, including PDE4 inhibitors, Janus kinase (JAK) inhibitors and others. So far, good evidence exists and there is promise with agents that inhibit IL-4 and/or IL-13, as well as other interleukins. Options modulating other targets lack evidence in the treatment of atopic dermatitis, according to the paper Are biologics efficacious in atopic dermatitis: A systematic review and meta-analysis, published in 2018 in the American Journal of Clinical Dermatology.
Researchers have shown that dupilumab, which blocks the alpha subunit of the IL-4 receptor thereby affecting signaling of the IL-4 and IL-13 pathways, significantly decreases signs and symptoms of atopic dermatitis without the need for laboratory monitoring. It appears to be relatively safe and doesn’t seem to increase infection risk. Dr. Del Rosso writes that in his opinion, “…based on the available data and experiences thus far, dupilumab therapy offers a more favorable overall safety profile in comparison with the available oral systemic agents.”
Dr. Dao says dupilumab, which is FDA approved to treat moderate-to-severe atopic dermatitis in adults and adolescents, is his therapy of choice in those patients with moderate-to-severe disease who fail conventional dry skin care, phototherapy, and/or topical prescription therapies.
RELATED: Dupilumab a gamechanger in pedatirc AD
“With no lab work monitoring required, it has been quite easy for me to convince my patients who needed dupilumab to give it a try. I feel unjust in not offering my patients that option, unless any contraindications exist such as hypersensitivity to dupilumab itself. As dupilumab has not been studied in pregnancy, I would also avoid dupilumab treatment in this population until we get more data on safety. Lastly, in patients with severe ocular manifestations of atopic dermatitis, I would work in conjunction with ophthalmology to coordinate care,” Dr. Dao says.
Dr. Dao says dupilumab has been game changer in practice.
“I once had a teenager with lifelong severe atopic dermatitis, and in my initial visit with her, she was erythrodermic, bleeding from head to toe from her fissured skin, shivering with her arms folded in the hallway. Her family refused dupilumab as it was a new treatment, and it was no surprise when she was still erythrodermic at her follow-up visit one month later,” Dr. Dao says. “Dupilumab for her was a game changer, as it has been for the majority of my patients who had started this new biologic treatment. I have come to expect my patients at their follow-up appointments to feel better and tell me that they do not even need any more topical steroid refills. Most of my patients with ocular side effects such as conjunctivitis have been well managed with my ophthalmology colleagues, including those with pre-existing atopic keratoconjunctivitis and those with de novo conjunctivitis after dupilumab initiation. It is difficult to erase decades of lichenification at the 2- to 3-month follow-up appointments, but I am hopeful that even the sequelae of decades of poor atopic dermatitis control will begin to fade over the years. The most important thing is that my patients feel like they have their life back and can concentrate on everything they have wanted to, besides their itchy skin.”
But Dr. Dao counsels patients with atopic dermatitis that dupilumab does not obviate dry skincare regimens and contact allergen avoidance.
“They will not fully realize benefits of treatment if they continue hot baths or shy away from regular use of their safe emollients, and I aggressively evaluate any suspicion of allergic contact dermatitis with patch testing as well,” Dr. Dao says. “After all, if there is a concomitant allergic contract dermatitis, dupilumab at best may just mask another fully avoidable process and complicate evaluation of improvement attributable to dupilumab therapy.”
Dr. Dao says that while dupilumab can be a great option for these patients, it isn’t for everyone.
“A big concern I have is if dupilumab becomes first line treatment for allergic contact dermatitis, or starts being used for mild cases of atopic dermatitis,” Dr. Dao says. “Traditional pursuits of patch testing to identify occult allergic contact allergens and taking time to counsel patients regarding dry skin care should not be forsaken with new biologic treatments for atopic dermatitis.”
Pipeline update
Monoclonal antibody IL-13 inhibitors lebrikizumab and tralokinumab are in development and early research suggests they’re promising options in the treatment of adults with moderate-to-severe atopic dermatitis. In the case of tralokinumab, research suggests that serum level of dipeptidyl peptidase 4 might be a predictive biomarker for which patients might benefit from tralokinumab therapy, according to Dr. Del Rosso.
“While we are not yet at the level of personalized medicine, targeted therapy is the next step in evolution of our medical treatments. With targeted therapy comes a large responsibility to continually monitor our patients, however, as we never can fully understand the implications of modulating complex signaling pathways,” Dr. Dao says.
Disclosures:
Dr. Del Rosso is a consultant, speaker, and/or researcher for several companies who market products used in the management of atopic dermatitis or have compounds under development. These include Almirall, Dermira, Galderma, Genentech, LaRoche Posay, Leo Pharma, Loreal, Ortho Dermatologics, Pfizer, Promius, Regeneron, Sanofi-Genzyme, Skinfix, Sonoma, Sun Pharma, and Taro.
Dr. Dao reports no conflicts of interest on this topic.







