- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Guidance on transitioning psoriasis patients between biologics
Author(s):
Switching patients with moderate-to-severe plaque psoriasis from one biologic to another is a multifactorial decision and may depend on safety, efficacy or dosing intervals. Ron Vender, M.D., offers insight into how to switch appropriately.
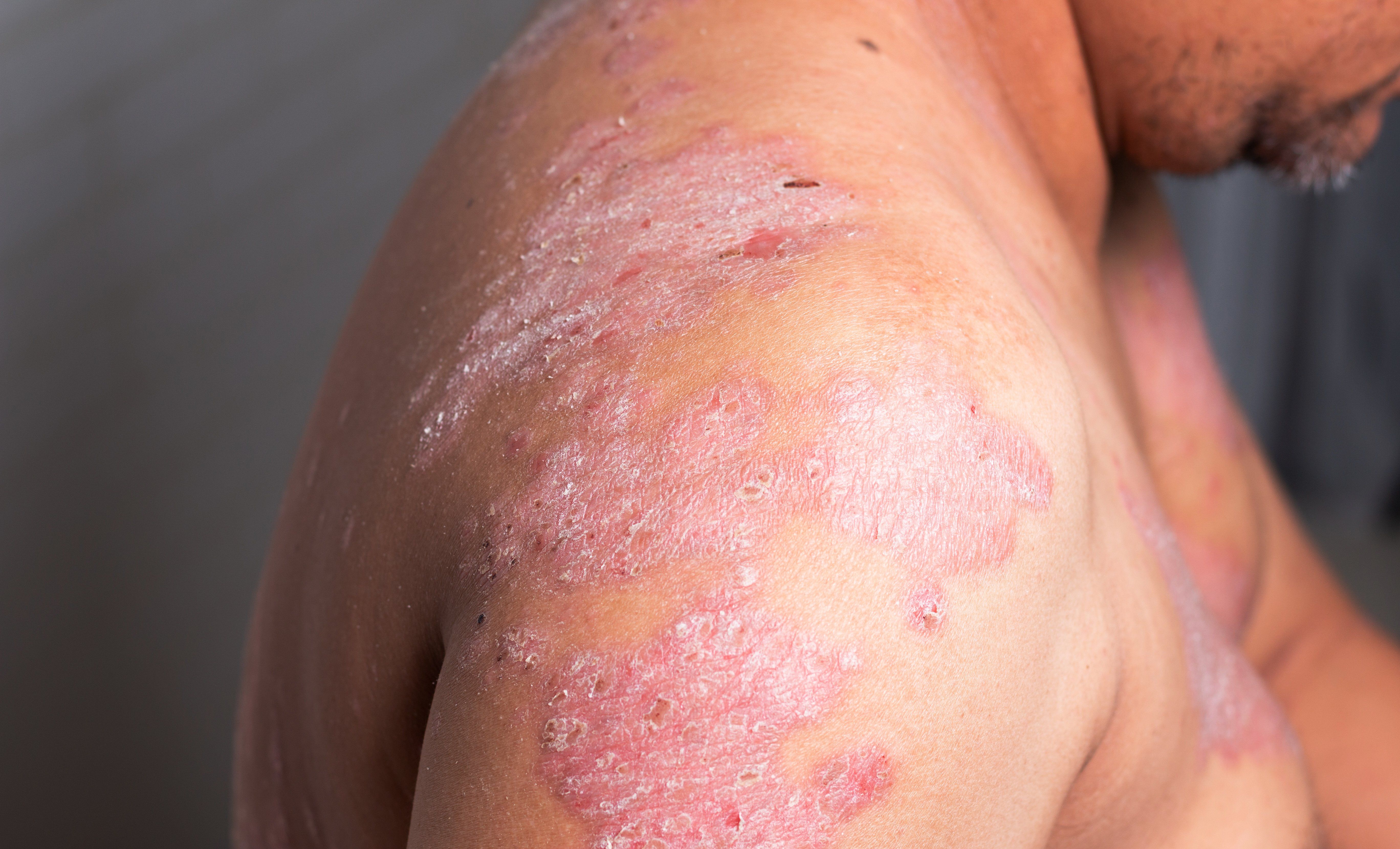
With many biologics now available for patients with moderate-to-severe plaque psoriasis, physicians may find themselves transitioning among them to optimize treatment and improve quality of life, according to Ron Vender, M.D., associate clinical professor of dermatology, McMaster University, and founder and director, Dermatrials Research, Inc, Hamilton, Ontario, Canada.
RELATED: Read about recent updates in topical therapies for psoriasis
Switching among agents is one strategy to consider when patients fail to achieve an adequate response to biologic therapy, experience secondary failure with loss of efficacy over time, or develop an adverse reaction, he says. Dose or dosing interval adjustments may also be considered. In addition, there are also reports that repeating the induction phase regimen can help patients who are secondary failures.
What constitutes treatment failure is another issue to consider. The high PASI 90 response rates that can be achieved with the newer biologics have raised the bar. Individual patients, however, will differ in their outcome expectations, and use of adjuvant topical or phototherapy might be reasonable interventions to address localized resistant plaques in patients who fail to clear.
“Before making a switch for primary or secondary failure, clinicians also need to rule out a role for treatment nonadherence,” Dr. Vender says.
Dr. Vender recently published a paper that aims to provide clinicians with practical guidance for changing biologic therapy in patients being treated for moderate-to-severe plaque psoriasis who are deemed efficacy failures or develop a safety concern.1 He summarizes findings from relevant literature and offers recommendations about how and what biologic to transition to given different clinical scenarios.
Dr. Vender tells Dermatology Times that he undertook reviewing and synthesizing the literature on transitioning between biologics because of the absence of randomized, placebo-controlled trials comparing transitioning to different biologics and the paucity of guidance about when to start a new biologic for patients who were not responding to or not tolerating their existing biologic therapy
RELATED: Biologic for psoriasis temporarily impacts aortic vascular inflammation
“I believe my paper provides a guide as to when switching a biologic agent for treatment of moderate-to-severe plaque psoriasis is appropriate as well as information that will help clinicians make safe and effective choices when making the switch,” Dr. Vender says.
Choosing the next therapy
As with primary treatment decisions, patient comorbidities are factored into selecting the next biologic therapy. For patients with psoriatic arthritis, a TNF-α inhibitor or IL-17A blocker would be preferred because those agents have indications for both psoriasis and psoriatic arthritis.
For patients with inflammatory bowel disease, another relatively common comorbidity in patients with psoriasis, adalimumab, certolizumab, infliximab, and ustekinumab are good choices since they are all approved for treatment of Crohn disease and/or ulcerative colitis, but IL-17 blockers should be avoided.
In patients with manual dexterity issues, consideration can be given to a biologic that can be administered with an auto-injector.
Otherwise, reports in the literature indicate that in cases of insufficient response, switching to another biologic within the same class or to a biologic in a different class can both be safe and effective strategies.
RELATED: New pediatric psoriasis guidelines offer management pearls
Timing the transition
Recommendations regarding the timing for starting a different biologic differ based on whether the switch is being made due to safety concerns or lack of efficacy. If a change is being made because the patient experienced an adverse event with existing biologic treatment, evidence supports waiting until the reaction has resolved or elevated laboratory parameters have normalized or stabilized.
In the situation where the reason for switching is lack of efficacy, Dr. Vender suggests beginning the new biologic when the existing therapy was next scheduled for administration or based on the minimal or shortest dosing interval for the failed agent’s induction schedule.
For example, in the case where a patient is failing treatment with etanercept (Enbrel, Amgen), a new biologic could be started one week after the last etanercept dose. The switch to a new biologic can be made two weeks after the last dose of adalimumab (Humira, Abbvie), infliximab (Remicade, Janssen CarePath), certolizumab (Cimzia, UCB), secukinumab (Cosentyx, Novartis), ixekizumab (Taltz, Eli Lilly), or brodalumab (Siliq, Valeant Pharmaceuticals), while a four-week delay before switching is suggested when treatment is being transitioned from ustekinumab (Stelara, Janssen CarePath), guselkumab (Tremfya, Janssen CarePath), tildrakizumab (Sun Pharma), or risankizumab (Skyrizi, Abbvie).
Available evidence seems to weigh against the need to wait for washout of the existing therapy, according Dr. Vender.
“The washout period for biologics is usually considered to be four half-lives,” he says. “Waiting that long to begin a new biologic can put patients at risk for worsening of their disease and quality of life, and the likelihood for that occurring appears to be greater than the likelihood that overlapping therapies will result in an adverse effect,” he explains.
Disclosures:
Dr. Vender has received grants and/or research support from companies that market biologics for treatment of plaque psoriasis. He received no financial support for the research, authorship, or publication of his article on transitioning between biologics.
References:
Vender R. Transitioning between biologics. Skin. 2019;3(6):374-80.






