- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Year-Long Analysis Reveals High Rates of Patient Satisfaction With OnabotulinumtoxinA Treatment
Author(s):
Allergan Aesthetics has shared details of a recently-published analysis involving patient-reported satisfaction, including natural-looking outcomes, with OnabotulinumtoxinA (Botox) treatment.
Allergan Aesthetics has announced1 high rates of patient-reported satisfaction with OnabotulinumtoxinA (Botox), or onabotA, treatment following a newly-published post-hoc analysis, including satisfaction with natural-looking outcomes.
hedgehog94/Adobe Stock
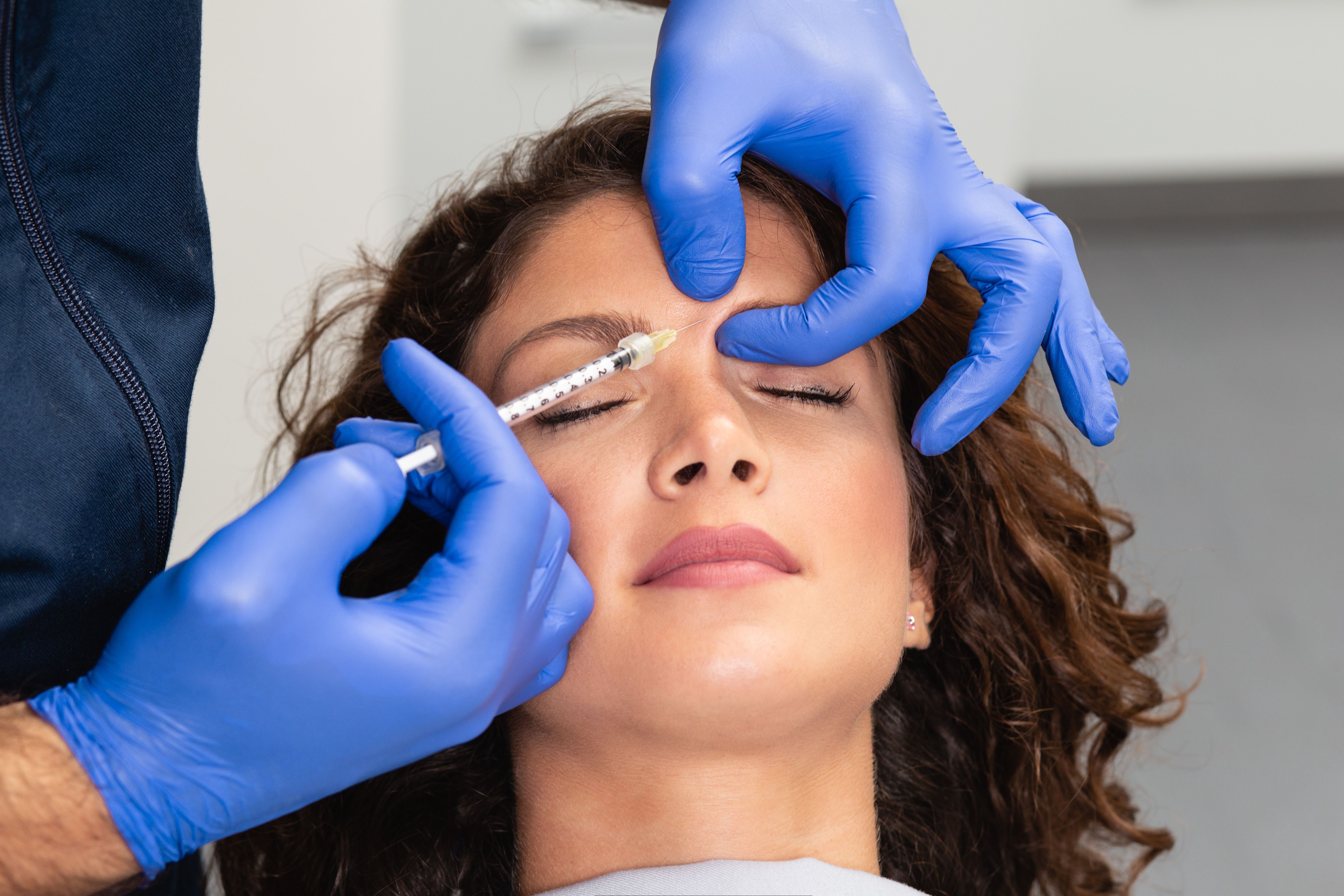
Tha analysis,2 published in the Journal of Cosmetic Dermatology, explored one of the most commonly reported barriers to treatment: fear of unnatural-looking outcomes.
"Regardless of age or gender, most patients contemplating facial aesthetic treatments desire refreshed, natural-looking results, and fear of looking unnatural or a 'frozen' look is a common barrier for many," said Steven Dayan, MD, in a press release from AbbVie.1 Dayan is founder of Denova Research and an author of the study.
"Self-perceived patient satisfaction, both in physical appearance and emotional wellbeing, is paramount in aesthetics medicine and it is vital physicians understand how patients perceive themselves after treatment to truly address patient concerns and help fulfill their expectations,” Dayan said.
Investigators pooled data from 2 phase 3 studies (NCT02261467 and NCT02261493), both of which were randomized, placebo-controlled, and included data from a 12-month period. Both studies explored the efficacy of onabotA in botulinum toxin naïve adults; study 1 evaluated patients with moderate to severe forehead lines and glabellar lines while study 2 evaluated patients with moderate to severe crow’s feet lines. Each involved randomization to treatment arms, which included varying dosages of onabotA or a placebo.
Both studies consisted of a 6-month double-blind period, 6-month open-label treatment period, and follow-up evaluations on days 7, 14, 30, and every 30 days after each treatment.
Patients in each study completed Facial Line Satisfaction Questionnaires (FLSQ) involving patient-perceived treatment satisfaction, psychological impact, and stand-alone items related to satisfaction. These included satisfaction with a natural look, the effect of treatment, and whether or not the treatment met or exceeded their expectations.
Investigators conducting the post-hoc analysis of these studies found that 30 days after receiving treatment with onabotA, 90.5% of participants reported being either mostly or very satisfied with the natural-looking outcomes of the treatment; this level of treatment satisfaction was sustained by 80% of participants during the year-long study period. Furthermore, more than half of participants also reported meaningful improvements to self-perceived appearance and overall psychological well-being.
"Assessing patient-reported outcomes for toxin treatments is an important advancement in supporting physicians with reliable data and education to help ensure patients achieve their expected and desired results," said John Maltman, vice president, global aesthetics medical affairs, AbbVie, in the press release.1 "The study supports that patient satisfaction and a desired natural look that helps improve the appearance of upper facial lines can be achieved with proven facial aesthetic neuromodulator treatment."
References
- Journal of Cosmetic Dermatology publishes data demonstrating patient satisfaction with natural-looking outcomes following treatment with Onabotulinumtoxina (Botox® cosmetic). News Center. August 30, 2023. Accessed August 30, 2023. https://news.abbvie.com/news/press-releases/journal-cosmetic-dermatology-publishes-data-demonstrating-patient-satisfaction-with-natural-looking-outcomes-following-treatment-with-onabotulinumtoxina-botox-cosmetic.htm?view_id=4667.
- Dayan S, Ogilvie P, Boyd C, et al. Self‐perception of natural outcome, appearance, and emotional well‐being after Onabotulinumtoxina treatment for upper facial lines: Post hoc analysis across age and gender. J Cosmet Dermatol. Published online 2023. doi:10.1111/jocd.15947





