- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
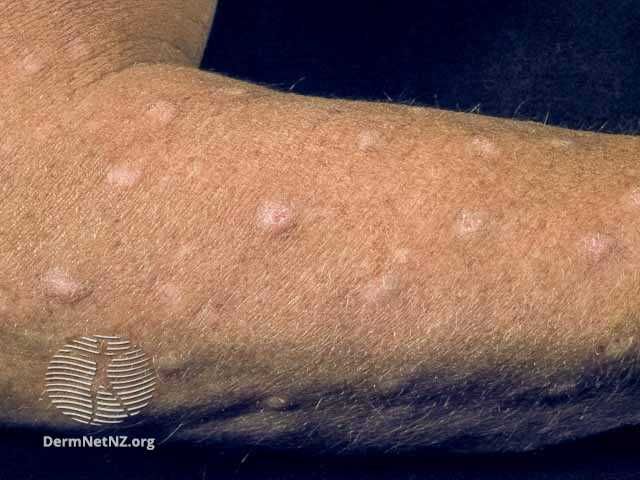
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Trevi Therapeutics Announces New Phase 2b/3 Results of Haduvio for Prurigo Nodularis
Author(s):
The continued reduction in WI-NRS was seen in patients who remained in the study through week 52.
Trevi Therapeutics recently announced new results from its phase 2b/3 PRISM open-label extension (OLE) study evaluating Haduvio (oral nalbuphine ER) for the treatment of prurigo nodularis. Data from PRISM was presented at the European Academy of Dermatology & Venereology Congress 2023 in Berlin, Germany.1
The primary endpoint of the study was the proportion of patients achieving a greater than or equal to 4-point improvement in the weekly mean Worst Itch Numerical Rating Scale (WI-NRS) score at week 14 compared to baseline. Patients who completed week 14 were eligible to roll into an additional 38-week OLE trial. During the 38-week OLE period, all patients received Haduvio 162mg twice a day. Overall, post hoc analyses showed a continued reduction in mean WI-NRS for patients who received Haduvio through week 52.
"We are pleased with the results from our preliminary analyses of our OLE data supporting the safety profile of nalbuphine ER up to 52 weeks which were presented at EADV today," said Jennifer Good, president and CEO of Trevi Therapeutics, in the news release. "The long-term data also supports the continued effectiveness of Haduvio in reducing itch over 52 weeks for the participants who remained in the study. We are preparing for an end of phase 2 meeting with the FDA to discuss next steps in development for this program."
In total, 151 patients completed the OLE. The safety data were consistent with the safety profile of Haduvioreported in the initial 14-week period of PRISM and previous trials of Haduvio. Adverse events (AEs) reported with a frequency greater than 5% in the 38-week open-label period included nausea, dizziness, vomiting, fatigue, and somnolence. Study discontinuation due to AEs occurred in 13% of patients during the open-label period, and serious AEs were reported in 13 patients. Only 2 of the reported serious AEs were considered potentially treatment-related.
PRISM (NCT03497975) was a randomized, double-blind, placebo-controlled study to evaluate the safety and efficacy of Haduvio in prurigo nodularis. Patients were randomized equally across 2 treatment groups: oral Haduvio 162 mg or placebo twice daily including an initial 2-week blinded titration period.
Haduvio is also being investigated for the treatment of difficult to treat patients with chronic cough in idiopathic pulmonary fibrosis and other chronic cough indications.
Reference
- Trevi Therapeutics announces results from phase 2b/3 PRISM open-label extension study of Haduvio in prurigo nodularis. PR Newswire. News release. October 13, 2023. Accessed October 13, 2023. https://www.prnewswire.com/news-releases/trevi-therapeutics-announces-results-from-phase-2b3-prism-open-label-extension-study-of-haduvio-in-prurigo-nodularis-301955819.html