- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
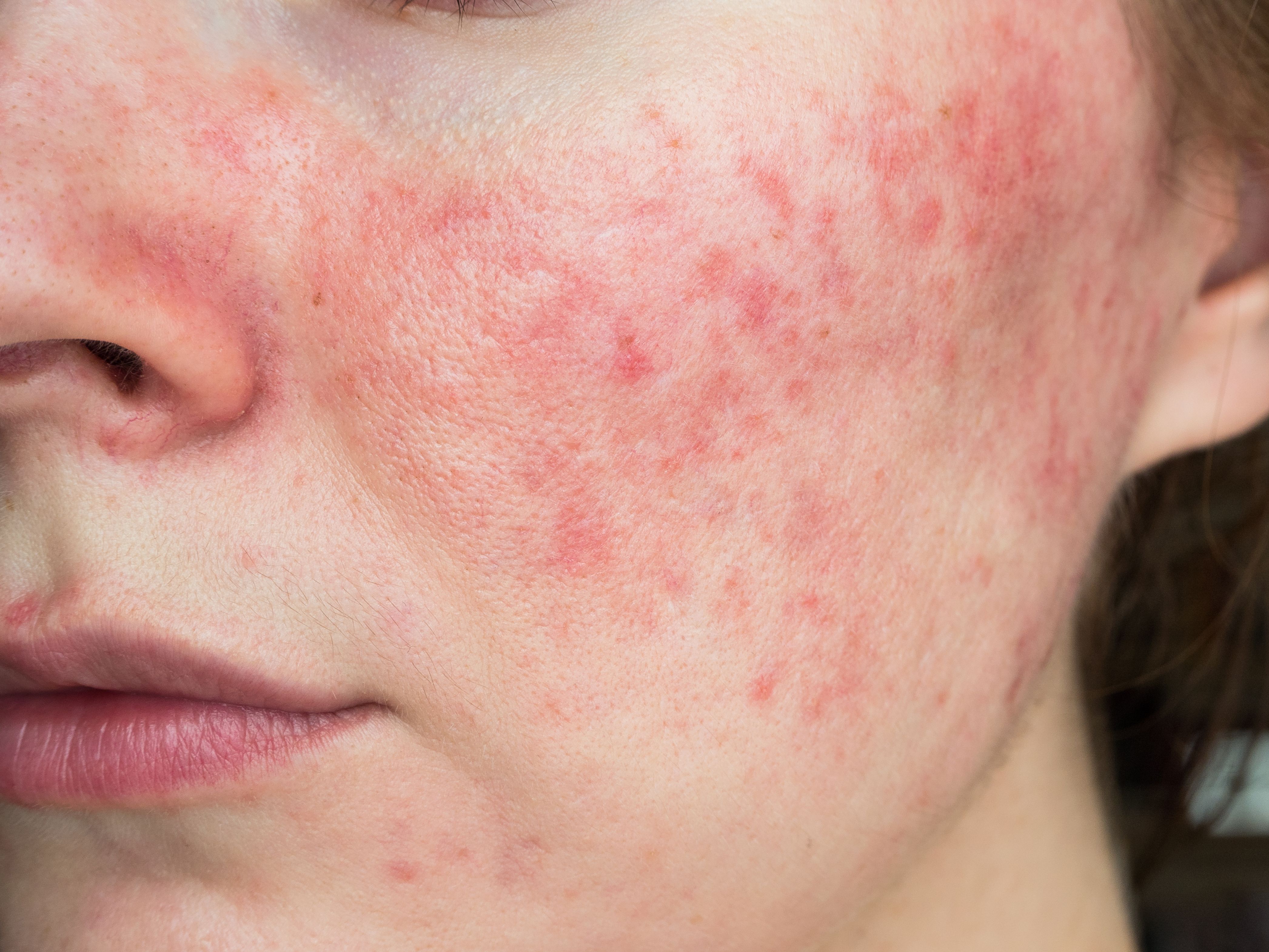
Topical minocycline for rosacea now available
Author(s):
ZILXI (FMX103, VYNE Therapeutics), a 1.5% topical minocycline foam, is now available for prescription for the treatment of inflammatory lesions of rosacea in adult patients.
A new treatment for inflammatory lesions of rosacea in adults is now on the market with the recent announcement from VYNE Therapeutics (formerly Menlo Therapeutics), that their newly United States Food and Drug Administration (FDA)-approved 1.5% topical minocycline foam, FMX103 (ZILXI, VYNE Therapeutics), is now available in pharmacies nationwide.
Minocycline is a broad-spectrum antibiotic within the tetracycline family that possess anti-inflammatory properties. FMX103 is specifically equipped with VYNE’s proprietary Molecule Stabilizing Technology (MST) that helps deliver the drug through a foam-based vehicle along with coconut and soybean oil for added moisturization, according to the company.1
MORE: FDA approves first topical minocycline for rosacea
In May 2020, FMX103 became the first FDA-approved topical minocycline treatment for rosacea, following positive results from two randomized, multicenter, vehicle-controlled, double-blind clinical trials that enrolled 1,522 patients 18 years and older.2
FMX103 met all co-primary endpoints in the studies, as well as demonstrated superiority over vehicle and a statically significant decrease in inflammatory lesion count and Investigators Global Assessment (IGA) success.2
RELATED: Menlo Therapeutics changes name to VYNE Therapeutics
"Patients and physicians have been seeking new treatment options for rosacea, a condition that can be difficult to treat, leaving many patients dissatisfied and, in some cases, switching treatments multiple times or discontinuing altogether," says David Domzalski, CEO of VYNE Therapeutics. "By combining a unique topical delivery system for minocycline with strong efficacy and tolerability, ZILXI is positioned to address a very challenging skin condition in a way that could change treatment considerations for rosacea."
VYNE announces the annual list price for FMX103 will be $485 per 30-gram canister. The company says they are currently working with commercial insurance companies to align contracts to help expand access to the topical.
References:
1. Inc., V. (2020, October 01). VYNE Therapeutics Announces ZILXI™ (minocycline) topical foam, 1.5% Available in Pharmacies Nationwide on October 1st. Retrieved October 01, 2020, from https://www.prnewswire.com/news-releases/vyne-therapeutics-announces-zilxi-minocycline-topical-foam-1-5-available-in-pharmacies-nationwide-on-october-1st-301143663.html?cc
2. Petronelli, M. (2020, May 29). FDA approves first topical minocycline for rosacea. Retrieved October 01, 2020, from https://www.dermatologytimes.com/view/fda-approves-first-topical-minocycline-rosacea

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.