- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
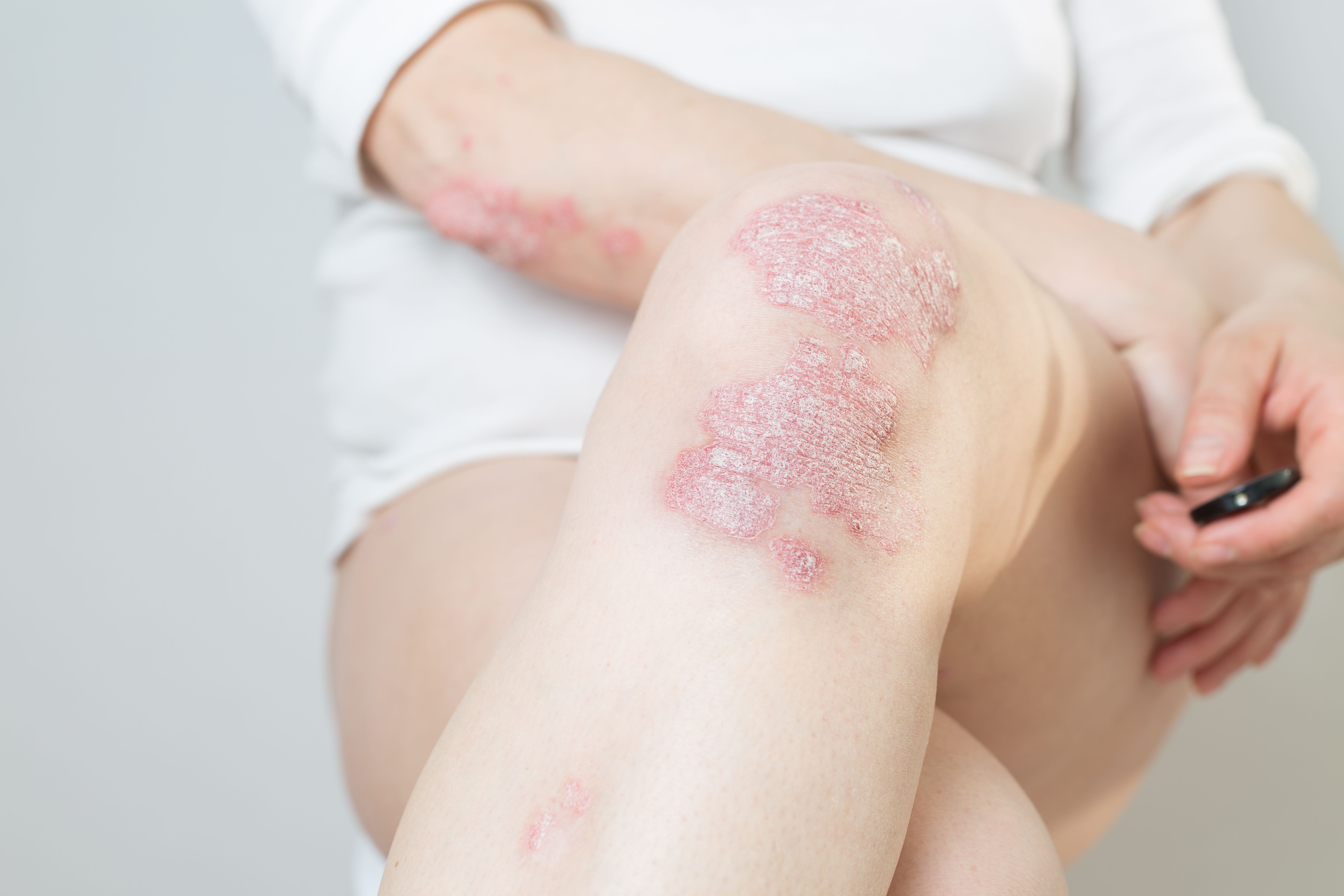
Topical Melatonin and Rosuvastatin Reduce Severity of Mild to Moderate Plaque Psoriasis
Author(s):
Versus placebo, topical melatonin and rosuvastatin reduced psoriatic plaque severity with a favorable safety profile.
Topical melatonin and rosuvastatin are capable of reducing the severity of mild to moderate plaque psoriasis, according to a study published in Skin Research and Technology.1 The study evaluated the therapeutic potential of these treatments versus a placebo.
Recent research in psoriasis has explored the therapeutic potential of topical formulations containing melatonin and rosuvastatin for disease management, according to study authors Mohammadi et al. Melatonin, known for its role in regulating circadian rhythms, exhibits anti-inflammatory and antioxidant properties, making it a promising candidate for psoriasis treatment, they wrote. At the site of wounds, melatonin has also demonstrated the ability to interfere with an increase in key players in inflammatory skin diseases: IL-12, TNF-α, and INF-γ.2
Rosuvastatin, primarily used as a lipid-lowering agent, has also demonstrated immunomodulatory and wound healing properties. These wound-healing properties, according to previous research, are mediated by increasing vascular endothelial growth factor and regulating angiogenesis.3
To evaluate the efficacy of these topical formulations, researchers conducted a randomized, controlled, double-blind clinical trial involving 77 patients with mild to moderate plaque psoriasis. Patients were required to be at least 18 years of age with a diagnosis of stable plaque psoriasis with less than 10% of the body's surface area affected by lesions or plaques.
Prior to treatment, all patients were required to partake in a 4-week washout period and were instructed to halt any use of psoriasis therapies such as phototherapy. Patients were assigned to receive either melatonin cream, rosuvastatin cream, or a placebo for a total of 8 weeks. The topical formulations were applied on a twice-daily basis, and all patients were asked to discontinue use of any other moisturizing products.
Utilizing scoring systems such as the Psoriasis Area and Severity Index (PASI), the Dermatology Life Quality Index (DLQI), and the Dermatology Severity Score (DSS), researchers made assessments of disease severity at baseline, day 30, and day 60.
Results indicated significant improvements in PASI scores, DSS, and DLQI in both the melatonin and rosuvastatin treatment groups compared to baseline. Melatonin cream demonstrated a moderate response, with a 51% reduction in PASI scores and significant improvements in DLQI and DSS. Similarly, rosuvastatin cream led to a 70% reduction in PASI scores and significant enhancements in DLQI and DSS.
Adverse effects were minimal, with mild itching and scaling reported in some patients receiving rosuvastatin cream. No secondary infections were observed during the treatment period.
Study limitations, as noted by researchers, included the small size of the subject groups, including a limited number of patients entering the study, and the exclusion of patients who did not correctly apply their intervention creams--particularly in the control group.
"[The] current study is the first double-blind randomized clinical trial evaluating the efficacy of each of topical melatonin or rosuvastatin alone in comparison to placebo in mild to moderate psoriasis," wrote Mohammadi et al. "The results of this clinical trial demonstrated that melatonin and rosuvastatin creams, 5% (w/w) were superior to the placebo cream in each of PASI, DSS and, DLQI scores. Topical melatonin and rosuvastatin diminished the severity of mild to moderate plaque psoriasis with a satisfactory safety profile. Future clinical trials should assess both the long-term efficacy and safety of melatonin and rosuvastatin creams in larger study populations."
References
- Mohammadi F, Harofteh FZ, Sahebnasagh A, Ghaneei N, Ardakani MEZ, Saghafi F. Efficacy and safety of topical rosuvastatin & melatonin vs. placebo in patients with mild to moderate plaque psoriasis: A preliminary randomized double-blinded clinical trial. Skin Res Technol. April 2, 2024. Accessed April 3, 2024. doi:10.1111/srt.13689
- Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J, A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013. Accessed April 3, 2024.
- Kureishi Y, Luo Z, Shiojima I, et al., The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000. Accessed April 3, 2024.