- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Tildrakizumab Data Reveals Significant Wellbeing Improvement in Patients With Plaque Psoriasis
Author(s):
Data presented in a poster presentation at the 2023 World Congress of Dermatology meeting in Singapore showed significant improvements to patient quality of life.
Tildrakizumab (Ilumetri; Almirall) use in patients with moderate to severe plaque psoriasis led to clinically significant improvements in overall wellbeing, quality of life, and skin symptoms, according to a poster presented at the 2023 World Congress of Dermatology meeting in Singapore.
Milan Lipowski/AdobeStock
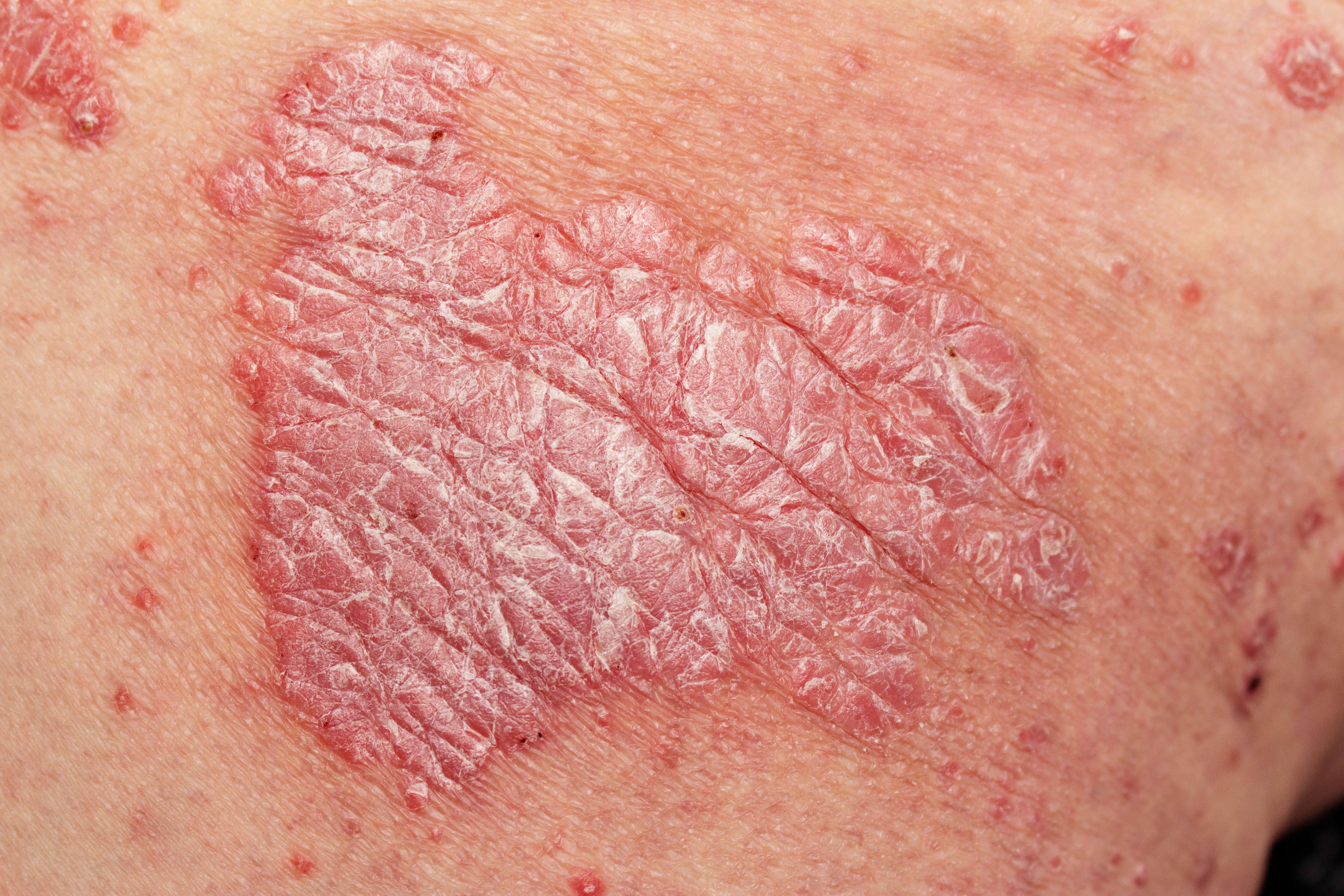
According to a press release from Almirall,1 results of a phase 3 study known as POSITIVE2 examined the real-world impact of tildrakizumab treatment in adult patients with more severe cases of plaque psoriasis.
Researchers sought to examine the effects of the drug, an interleukin-23p19 inhibitor, on overall patient wellbeing as well as its impact and long-term benefits on patient and physician satisfaction. They cited the known psychosocial impacts of psoriatic disease, noting that 77% patients with psoriasis in Europe felt their condition impacted their everyday life and wellbeing.
Other studies utilizing the Psoriasis Area and Severity Index (PASI), Physician Global Assessment, or Dermatology Life Quality Index, they said, limit researchers’ and physicians’ understandings of the disease perspective of patients. The non-interventional, prospective, observational, and real-world study is the first dermatologic clinical study to instead utilize the 5-item World Health Organization (WHO) WHO-5 Wellbeing Index as a primary tool to assess patient wellbeing.
Secondary endpoints were measured using the Physician’s Satisfaction Questionnaire, FamilyPso questionnaire (disease impact on patient partners), DLQI-R (skin-generic quality of life), TSQM-9 (treatment satisfaction), PBI-S-10 (treatment-related benefits), WPAI:PSO (work impairment), HRQoL (health-related quality of life), and absolute PASI score.
338 adult patients with a moderate to severe psoriasis diagnosis with a need for systemic biologic therapy who qualified for IL-23p19 treatment were selected for participation in the study. However, 780 patients have been enrolled in the ongoing study in total.
The study consisted ofa retrospective data collection period followed by a 24-month prospective data collection period, which included follow-up visits at 16 and 28 weeks, as well as 12, 18, and 24 months, post treatment.
Preliminary analysis of the study data has shown that tildrakizumab made significant improvements to patients’ overall quality of life and wellbeing with high rates of patient satisfaction reported. Furthermore, no new safety signals were reported. Participants were able to achieve a similar wellbeing status to that of members of the general population without psoriasis with as few as 16 weeks of treatment.
“We are thrilled with the promising results of the POSITIVE study, as they underline the importance of incorporating wellbeing into clinical trials,” said Frida Dunger Johnsson in the press release. Dunger Johnsson is the executive director of the International Federation of Psoriasis Associations, or IFPA. “Having a holistic and patient-centered approach allows for more data on the impact of treatments on people living with psoriatic disease and therefore improves health and quality of life.”
References
- Almirall: Ilumetri® (tildrakizumab) significantly improved the wellbeing of patients with moderate-to-severe plaque psoriasis. Business Wire. July 7, 2023. Accessed July 7, 2023. https://www.businesswire.com/news/home/20230707896992/en/Almirall-Ilumetri%C2%AE-tildrakizumab-significantly-improved-the-wellbeing-of-patients-with-moderate-to-severe-plaque-psoriasis.
- Augustin M, Sommer R, Daudén E, et al. Patient-reported well-being in value-based care using tildrakizumab in a real-world setting: protocol of a multinational, phase IV, 1-cohort prospective observational study (the POSITIVE study) BMJ Open 2023;13:e060536. doi: 10.1136/bmjopen-2021-060536






