- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Systemic nonbiologic therapies guidelines reflect continued popularity of oral treatments
Author(s):
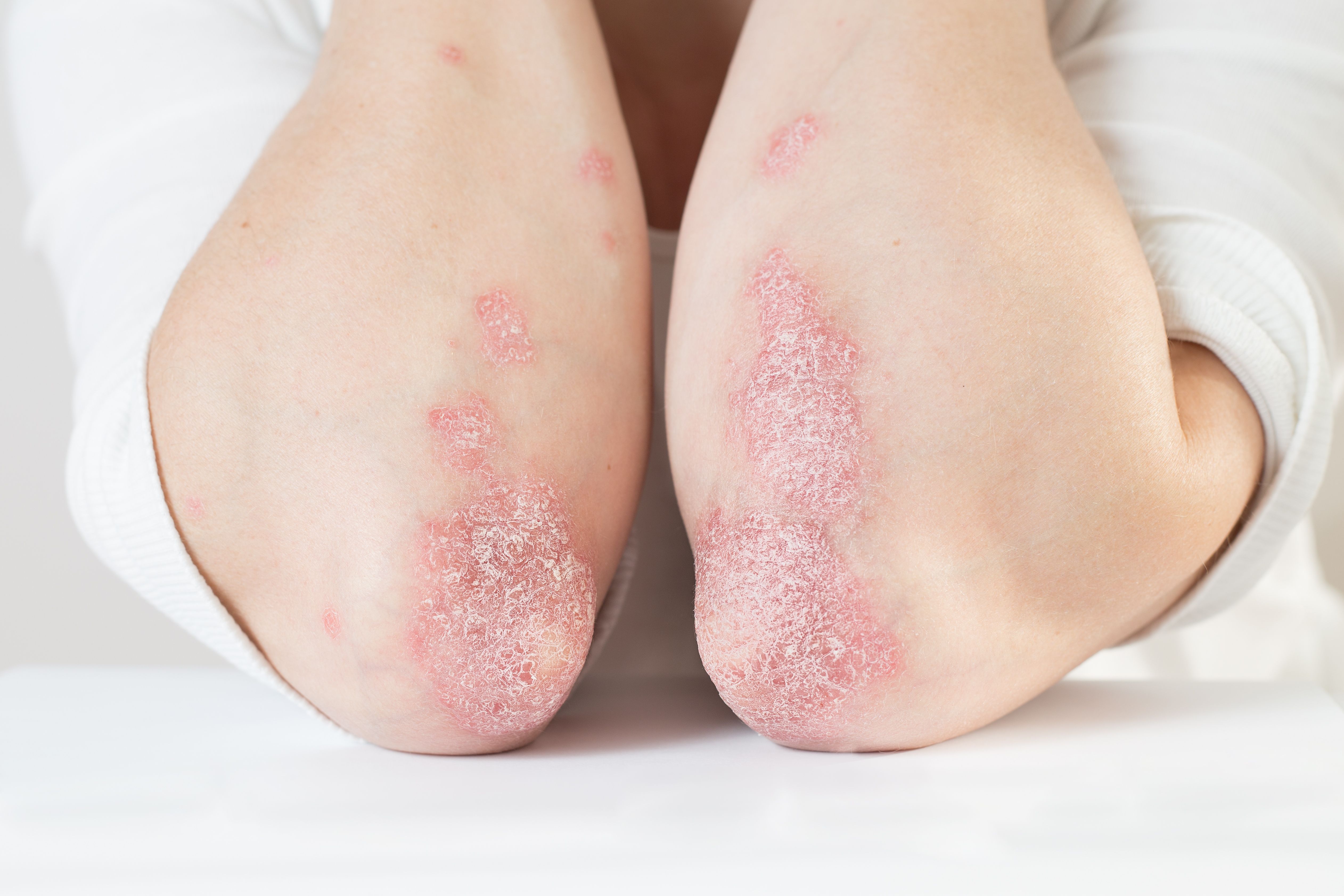
Guidelines on systemic therapies for psoriasis, developed jointly by the American Academy of Dermatology (AAD) and National Psoriasis Foundation, incorporate newer psoriasis medications and an evolving understanding of older ones.
“Oral therapies are still very commonly used. Many patients prefer oral psoriasis therapy to injectable biologic therapy,” says lead guideline author M. Alan Menter, M.D. He is chairman of dermatology at Baylor Scott & White Health, Dallas, co-chair of the AAD Psoriasis Guideline Workgroup and founder of the International Psoriasis Council.
Rheumatologists frequently prescribe methotrexate for psoriatic or rheumatic joint disease, either as monotherapy or in combination with biologic drugs. “When biologic drugs are not working as well as they should,” Dr. Menter says, “many dermatologists and most rheumatologists will add methotrexate in relatively low dosages and adjust the dose according to the response.”
Previous guidelines recommended considering liver biopsy after 3.5 to 4 cumulative grams of methotrexate. Instead, the new guidelines recommend noninvasive serologic testing and vibration-controlled transient liver elastography.
Supplementing the above tests with type III serum procollagen testing has been proposed as the ideal method of monitoring for methotrexate hepatotoxicity. However, this test has limited U.S. availability and has not been proven to eliminate the risk of serious liver complications. Menter et al. therefore recommend magnetic resonance elastography as a more accurate technique to consider in the event of technical failure with vibration-controlled transient elastography, or in patients at high risk of such failure.
Alternatively, Dr. Menter says that he refers patients to a liver expert for scintigraphy, which he says is as accurate as liver biopsy. “I have patients who have taken methotrexate for 10 to 15 years at a total amount of 5 to 8 g. They see him every two years. Liver biopsies are starting to fade away.”
Otezla (apremilast, Amgen) earned FDA approval for psoriasis in 2018. Apremilast provides Psoriasis Area and Severity Index (PASI) 75 response in one-third of patients. “But it’s a very safe drug with almost no side effects except for the fact that about 20% of people get gastrointestinal side effects for the first month.”
Among Janus kinase (JAK) inhibitors, the guidelines say, dermatologists can consider tofacitinib for moderate-to-severe psoriasis, although it is not FDA approved for this indication. In phase 3, a 15 mg BID dose achieved Psoriasis Area and Severity Index (PASI) 75 in 60% to 70% of patients, says Dr. Menter, lead investigator on the study. Due to concern over hematological side effects, however, the FDA required investigators to reduce the dosage to 10 mg BID, and thereafter to 5 mg BID.
“As a result, the new PASI 75 scores were in the 40% range. Tofacitinib is approved for rheumatoid arthritis and psoriatic arthritis, but it was never approved for psoriasis.” Rheumatologists like the drug for psoriatic joint disease, says Dr. Menter, often in place of methotrexate in combination with biologics.
Oral agents available outside the United States include fumaric acid esters (FAEs), which are FDA-approved for other indications including psoriatic arthritis. Dimethyl fumarate is FDA-approved for multiple sclerosis and can be recommended for psoriasis, guidelines state. Starting at low doses (one pill, 105 mg FAE mixtures) and escalating over eight weeks to six regular-strength pills (250 mg) as tolerated helps reduce gastrointestinal side effects.
Future guidelines will need to address systemic nonbiologic therapies that have completed phase 2 trials and likely will earn FDA approval for psoriasis in one to two years, adds Dr. Menter. Specifically, various tyrosine kinase 2 (Tyk2) inhibitors and JAK inhibitors typically provide clearance in 70% of patients. “I wouldn’t say we’ve reached our peak with biologic drugs. But we will be very happy with some of the new systemic oral drugs that will be coming to market.”
Disclosures:
Dr. Menter has been a consultant, investigator, speaker and/or advisory board member for AbbVie, Afecta Pharmaceuticals, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, Janssen, LEO Pharma, Menlo Therapeutics, Medimetriks Pharmaceuticals, Merck & Co., New Enterprise Associates, Novartis, Pfizer, Promius, Sienna Biopharmaceuticals, Spherix Global Insights US, UCB, Valeant Pharmaceuticals North America and Wyeth Labs.
References:
Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020 Feb 28. pii: S0190-9622(20)30284-X. doi: 10.1016/j.jaad.2020.02.044. [Epub ahead of print]