- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Study Reveals Comparable Efficacy of Clascoterone, Trifarotene, and Tazarotene in Acne Treatment
Author(s):
Key Takeaways
- Clascoterone, trifarotene, and tazarotene showed significant efficacy in reducing acne lesions compared to placebo in phase 3 RCTs.
- No significant differences in efficacy were found among the three treatments, allowing clinicians flexibility in choosing based on patient-specific factors.
The recent meta-analysis revealed comparable efficacy in treating acne vulgaris and was presented at Fall Clinical 2024.
Image Credit: © praisaeng
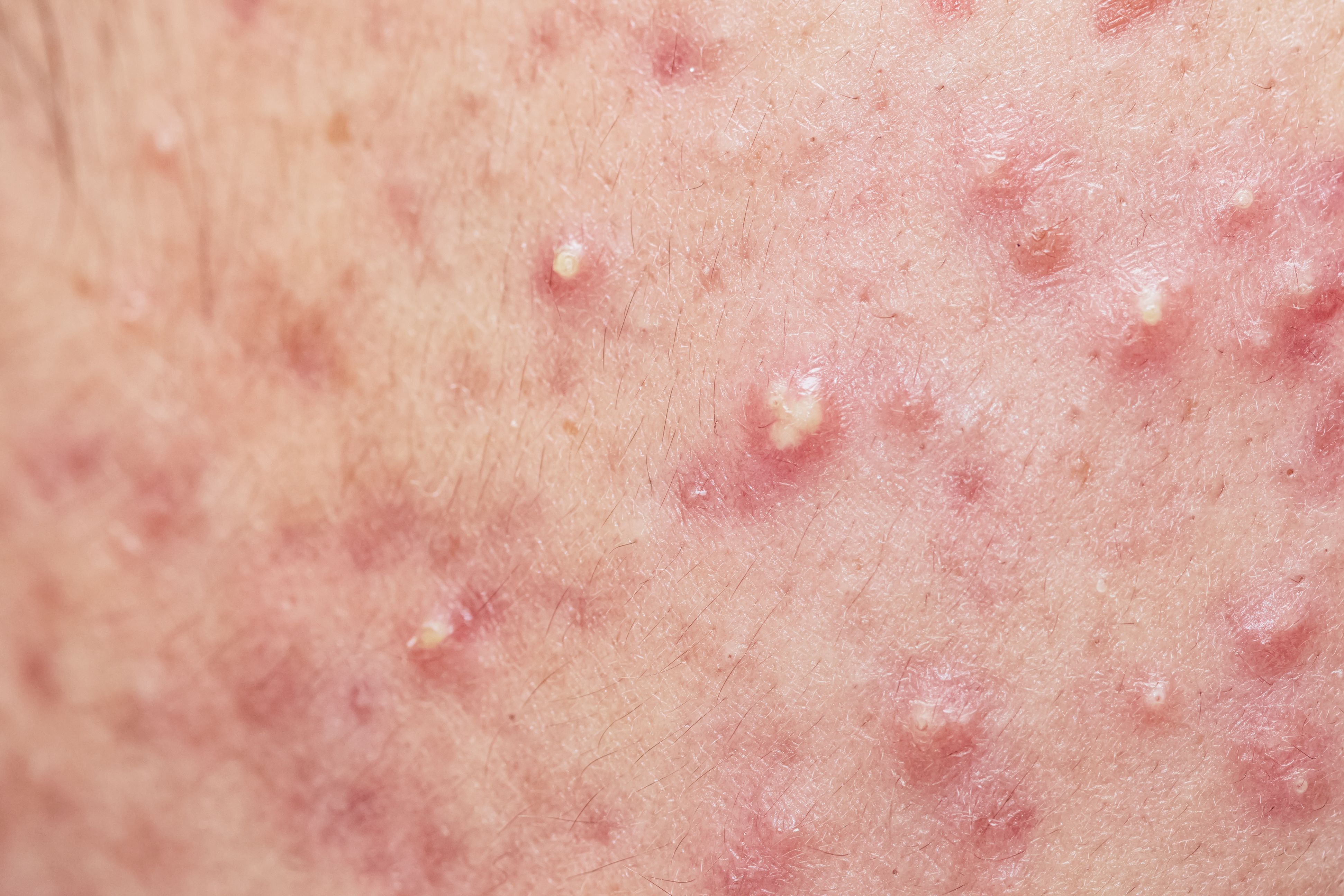
Sun Pharmaceutical Industries presented findings from a systematic review and meta-analysis at the 2024 Fall Clinical Dermatology Conference. The data, showcased in a poster,1 compared the efficacy of clascoterone (Winlevi; Sun Pharma), trifarotene (Aklief; Galderma), and tazarotene (Arazlo; Ortho Dermatologics) in treating moderate to severe acne vulgaris after 12 weeks of treatment.
Researchers conducted a systematic literature review to identify relevant randomized controlled trials (RCTs) comparing clascoterone cream (1%), trifarotene cream (0.005%), and tazarotene lotion (0.045%) against placebo. The comprehensive search of the Embase and MEDLINE databases, focusing on studies published up to October 2023.
Included trials reported on three primary outcomes: the mean percent reduction in inflammatory lesion count (ILC), the mean percent reduction in non-inflammatory lesion count (NILC), and the percentage of patients achieving treatment success at 12 weeks.
The systematic review identified 6 unique phase 3 RCTs involving a total of 5,474 patients. Among these, 2,735 were randomized to receive active treatment (clascoterone: 722 patients; trifarotene: 1,214 patients; tazarotene: 799 patients), while 2,739 were assigned to vehicle treatment.
The trials demonstrated that all 3 agents significantly reduced both inflammatory and non-inflammatory lesion counts compared to placebo.
The meta-analysis also revealed no statistically significant differences in efficacy among the 3 treatments. Specifically, the mean percent reduction in ILC and NILC, as well as the rates of treatment success, were comparable across clascoterone, trifarotene, and tazarotene.
The odds ratios for treatment success indicated that clascoterone had an OR of 2.90 (95% CI: 1.82, 4.61), trifarotene had an OR of 1.92 (95% CI: 1.57, 2.36), and tazarotene had an OR of 2.13 (95% CI: 1.67, 2.73). These findings suggest that while all 3 agents are effective, they exhibit similar efficacy profiles in reducing acne lesions.
According to poster authors Shergill et al, the lack of significant differences in efficacy among clascoterone, trifarotene, and tazarotene suggests that clinicians may choose any of these agents based on individual patient needs, preferences, and potential side effects.
However, they noted that the study primarily focused on efficacy, and safety profiles were not assessed in detail.
"Additional comparisons of safety outcomes between these agents will be important to determine if significant differences exist and to inform treatment decisions in clinical settings," wrote Shergill et al.
For more news from Fall Clinical, subscribe to receive our eNewsletter.
Reference
- Shergill M, Ali MU, Abu-Hilal M. Comparison of the efficacy of clascoterone vs trifarotene and tazarotene for the treatment of acne vulgaris at week 12: a systematic review and meta-analysis. Presented at: Fall Clinical 2024; October 24-27, 2024; Las Vegas, Nevada.






