- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
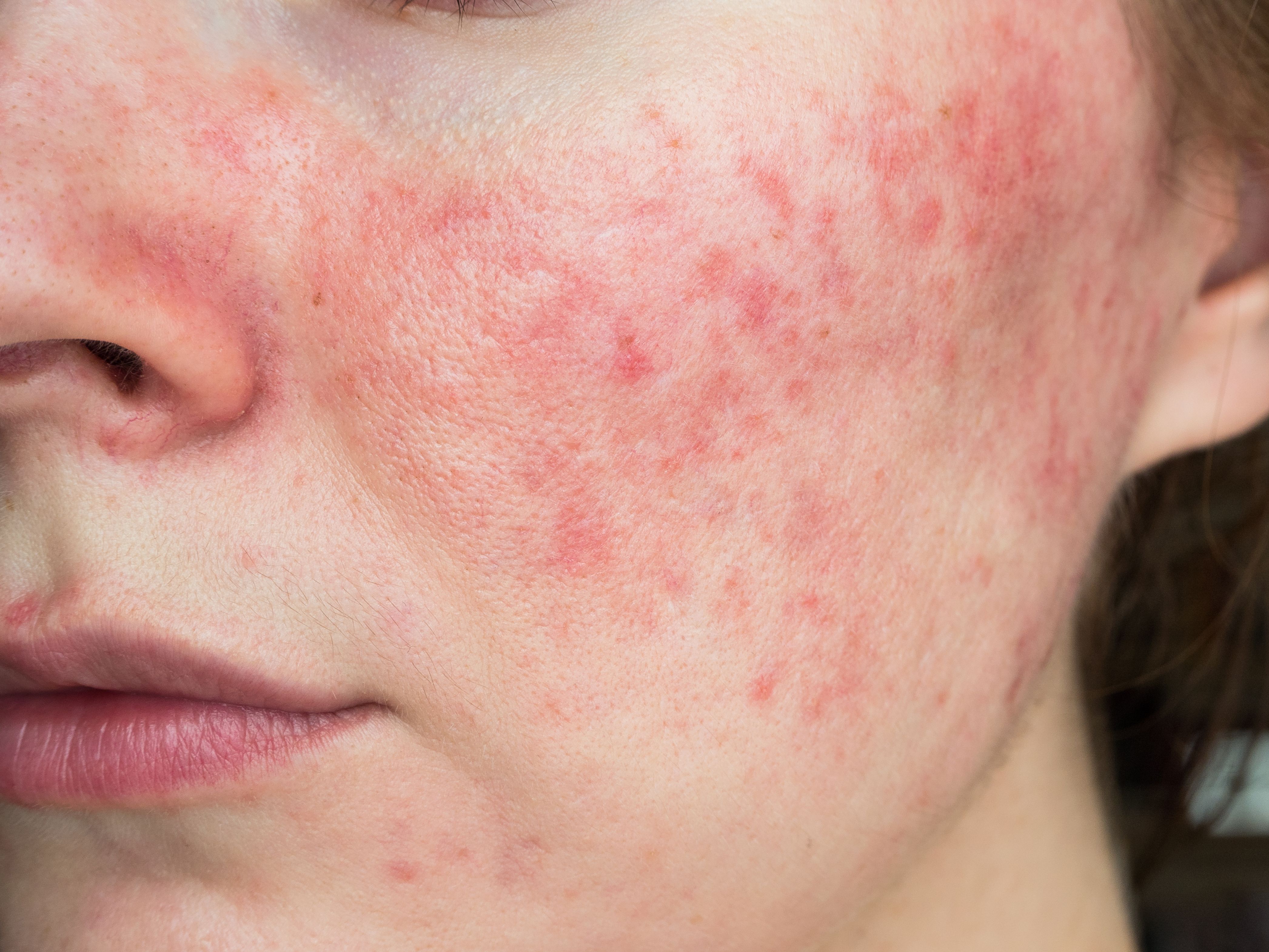
Study Investigates BTX Dosage for Rosacea Treatment
Author(s):
The study found that higher doses of BTX “significantly” improved facial erythema and patient quality of life compared to lower doses.
Image Credit: © Alessandro Grandini - stock.adobe.com

Because the causes and mechanisms of rosacea are not fully understood,it can prove challenging to manage. Patients frequently experience recurrent episodes, impacting their quality of life and leading to higher rates of embarrassment, social anxiety, and depression.1 Effective treatments are limited, especially for those with erythema telangiectasia due to their impaired skin barrier and sensitivity.2
Recent studies suggest that abnormal neurovascular regulation involving neuropeptides like substance P and vasoactive intestinal peptide (VIP) may play a role in rosacea.3 Botulinum toxin type A (BTX), known for its muscle relaxant properties and ability to inhibit the release of VIP and acetylcholine, has shown promise in reducing rosacea symptoms such as facial erythema and flushing.4 Despite its potential, the optimal dosage for BTX in treating rosacea varies, and there is a lack of standardized recommendations.
In light of this, a recent study aimed to evaluate the effectiveness and safety of different BTX doses in treating rosacea by conducting split-face trials to determine the best dosage for improving symptoms and patient quality of life.5
Study Methods and Treatment Plans
This single-center, randomized, double-blind, split-face clinical study involved 26 patients with facial erythema rosacea. Each cheek was randomly assigned to receive 0.5 U or 1 U of BTX. During the 12-week study, treatments for rosacea were restricted. Patients received lidocaine cream for anesthesia, followed by BTX injections into marked areas on one cheek, with 25 to 30 injection points per cheek. Post-treatment, patients were advised to avoid high temperatures and specific medications. The study assessed participants using the clinical erythema score (CEA), global aesthetic improvement scale (GAIS), dermatology life quality index (DLQI), VISIA red area measurements, and adverse events at 0, 2, 4, 8, and 12 weeks. Researchers stated recurrence was assessed at the 12-week follow-up.
Results
A total of 26 patients (2 men and 24 women) with telangiectasia rosacea participated in the study, ranging in age from 23 to 51 years, with a mean age of 33.2 years. Researchers found the CEA showed greater improvement with 1 U BTX compared to 0.5 U BTX, particularly at 2 to 8 weeks post-treatment. They stated the GAIS scores also indicated better outcomes with 1 U BTX at weeks 4, 8, and 12.
The mean red area absolute values, measured by VISIA, were significantly lower for the 1 U BTX-treated side at week 4 compared to the 0.5 U BTX-treated side. Researchers found the DLQI improved significantly from weeks 2 to 12 following BTX treatment. At the 12-week follow-up, the study stated relapse rates showed significant differences from baseline for both dosages, with the 1 U BTX-treated cheek showing greater improvement compared to the 0.5 U side.
In terms of safety, except for 1 patient who experienced transient facial tightness and 3 others with temporary erythema, no major adverse events were reported. Most patients only noted injection pain at the time of the procedure.
Conclusion
The study assessed the efficacy, safety, and optimal dose of BTX in treating rosacea by comparing 1 U and 0.5 U per injection point. The results indicated that a higher dose of 1 U per spot is more effective than 0.5 U in reducing facial erythema and enhancing patient quality of life. Despite the small sample size, researchers stated the study showed intradermal BTX can be a valuable and safe treatment option for rosacea, with 1 U doses proving to be more effective.
References
- Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71(5):973-980. doi:10.1016/j.jaad.2014.05.036
- Schaller M, Almeida LMC, Bewley A, et al. Recommendations for rosacea diagnosis, classification and management: update from the global rosacea consensus 2019 panel. Br J Dermatol. 2020;182(5):1269-1276. doi:10.1111/bjd.18420
- Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):53-62. doi:10.1038/jidsymp.2011.6
- Choi JE, Werbel T, Wang Z, Wu CC, Yaksh TL, Di Nardo A. Botulinum toxin blocks mast cells and prevents rosacea like inflammation. J Dermatol Sci. 2019;93(1):58-64. doi:10.1016/j.jdermsci.2018.12.004
- Jiang Y, Wang F, Chen W, et al. Assessing the efficacy and safety of intradermal injection of different doses of botulinum toxin type a: A randomized, double-blind, placebo-controlled, split-face pilot study in rosacea patients with erythematic telangiectasia. Dermatol Ther. 2024;2024:5596030. doi:10.1155/2024/5596030