- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Study Becomes First to Demonstrate Efficacy of Topical Isoniazid in Melasma
Author(s):
Study participants in the treatment group experienced an observable and significant decrease in melanin index scoring.
In the first known study to do so, a clinical trial of topical isoniazid demonstrated efficacy in the treatment of melasma. The study, published in the Journal of Cosmetic Dermatology, found that a treatment regimen including a topical formulation of isoniazid resulted in significant decreases in melanin index scoring.1
Researchers Ahramiyanpour et al noted that a lack of safe and effective depigmenting agents in melasma, coupled with safety concerns surrounding gold standard treatment hydroquinone, pose treatment challenges and limitations.
Hydroquinone is considered a controversial therapeutic option, with previous research posing potential concerns of mutagenic and carcinogenic properties. Some countries and regions, such as the United Kingdom, Australia, and Japan, have banned cosmetic ingredients containing hydroquinone.2
Prior research is suggestive of targeting the peroxidase enzyme in melanocytes as a way to develop skin-depigmenting agents. Researchers have proposed that isoniazid, a substance that is a peroxidase substrate, could be metabolized by melanocyte peroxidase, potentially reducing hyperactive melanocytes in the skin.3
With this information and context in mind, researchers sought to assess the therapeutic effects of topical isoniazid in patients with melasma.
Researchers conducted a prospective, double-blind, randomized controlled clinical at an outpatient dermatology clinic in Kerman, Iran. The study spanned from January 2021 to February 2022 and enrolled 20 female patients, aged 20 to 60, with confirmed diagnoses of melasma.
Patients with dermal or mixed type melasma were excluded, with the research focusing solely on epidermal melasma. Researchers documented demographic profiles and clinical histories for each study participant. This, coupled with the completion of comprehensive dermatological examinations and the utilization of tools such as clinical photography and colorimetric evaluations, were to objectively measure treatment efficacy and to assess the severity and anatomical distribution of melasma lesions.
Patients were randomized into treatment and control groups, with the former receiving topical 10% isoniazid while the latter received a placebo. Over a 3-month treatment period, participants were instructed to apply the assigned treatments while researchers monitored for adverse events.
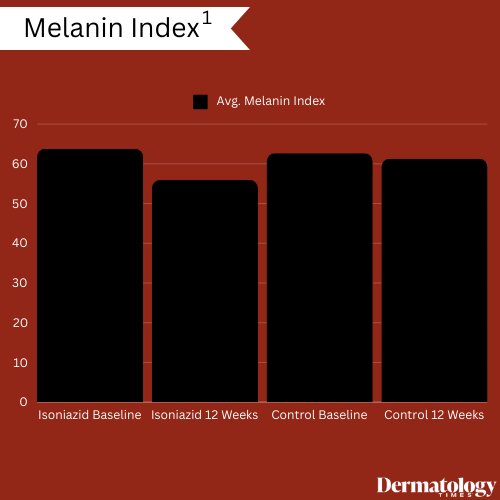
As a result, researchers noted significant reductions in melanin index, with an initial average score of 63.77 ± 6.27 among patients in the isoniazid treatment group at baseline to 55.92 ± 5.79 at the treatment period's conclusion, with gradual decreases observed every 4 weeks. While members of the control group experienced reductions in average melanin index scoring (62.65 ± 2.23 at baseline versus 61.25 ± 2.34 at conclusion), this reduction was not nearly as significant.
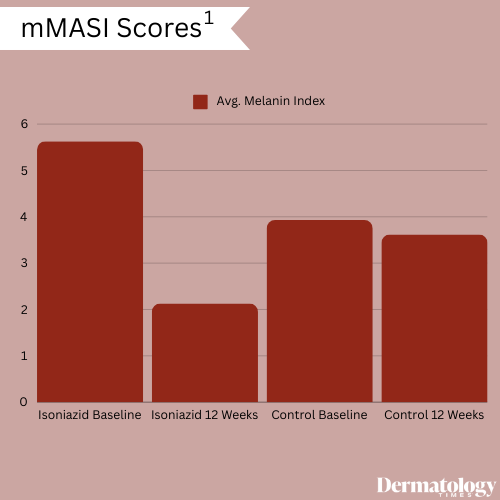
Members of both treatment groups also experienced improvements in modified Melasma Area and Severity Index (mMASI) scores, with patients treated with isoniazid experiencing significant reductions. At baseline, members of the treatment group had an average 5.63 ± 3.28 mMASI score; this reduced to 2.13 ± 1.71 following 12 weeks of treatment. At baseline, members of the control group recorded an average 3.93 ± 1.63 mMASI score with a 3.62 ± 1.74 score recorded at the study's conclusion.
In the treatment group, significant improvements were observed, with 4 patients experiencing excellent improvement, 3 very good improvement, and 3 good improvement, whereas most patients in the control group showed no or minimal improvement.
Quality of life, as measured by the MELASQOL score, significantly improved in the treatment group compared to the control group throughout the trial. No significant changes were observed regarding erythema in members of either the treatment or the control groups.
Adverse effects were minimal in the treatment group, with mild scaling, burning, and erythema reported in some patients, none of whom discontinued treatment due to side effects.
"Further clinical trials are necessary to confirm our results and determine the safety of the long-term use of isoniazid in treating melasma. Our study indicates that isoniazid offers promise as a novel skin depigmenting agent," according to study authors Ahramiyanpour et al. "While the mechanism of action of isoniazid is thought to involve its metabolism by melanocyte peroxidase to toxic metabolites, isoniazid may act as an inhibitor of melanosomal transfer because of its similarity of structure to other melanosomal transfer inhibitors such as niacinamide."
References
- Ahramiyanpour N, Mahmoudi Z, Nezhad NZ, Khazaeli P, Amiri R, Kasraee B. Topical isoniazid as a novel treatment for melasma: A randomized, double-blind, vehicle-controlled clinical trial. J Cosmet Dermatol. April 6, 2024. Accessed April 10, 2024. doi:10.1111/jocd.16320
- Juliano CCA. Spreading of dangerous skin-lightening products as a result of colourism: A review. Applied Sciences. 2022. Accessed April 10, 2024. https://doi.org/10.3390/app12063177
- Kasraee B. Peroxidase-mediated mechanisms are involved in the melanocytotoxic and melanogenesis-inhibiting effects of chemical agents. Dermatology. 2002; 205(4): 329-339. doi:10.1159/000066439







